Fire Extinguisher
Background
The hand-held fire extinguisher is simply a pressure vessel from which is expelled a material (or agent) to put out a fire. The agent acts upon the chemistry of the fire by removing one or more of the three elements necessary to maintain fire—commonly referred to as the fire triangle. The three sides of the fire triangle are fuel, heat, and oxygen. The agent acts to remove the heat by cooling the fuel or to produce a barrier between the fuel and the oxygen supply in the surrounding air. Once the fire triangle is broken, the fire goes out. Most agents have a lasting effect upon the fuel to reduce the possibility of rekindling. Generally, the agents applied are water, chemical foam, dry powder, halon, or carbon dioxide (CO 2 ). Unfortunately, no one agent is effective in fighting all types (classes) of fires. The type and environment of the combustible material determines the type of extinguisher to be kept nearby.
History
Fire extinguishers, in one form or another, have probably postdated fire by only a short time. The more practical and unitized extinguisher now commonplace began as a pressurized vessel that spewed forth water, and later, a combination of liquid elements. The older extinguishers comprised cylinders containing a solution of baking soda (sodium bicarbonate) and water. Inside, a vessel of sulfuric acid was positioned at the top of the body. This design had to be turned upside down to be activated, so that the acid spilled into the sodium bicarbonate solution and reacted chemically to form enough carbon dioxide to pressurize the body cylinder and drive out the water through a delivery pipe. This volatile device was improved by placing the acid in a glass bottle, designed to be broken by a plunger set on the top of the cylinder body or by a hammer striking a ring contraption on the side to release the acid. Cumbersome and sometimes ineffective, this design also required improvement.
Design
Aside from using different agents, manufacturers of extinguishers generally use some type of pressurized vessel to store and discharge the extinguishing agent. The means by which each agent is discharged varies. Water fire extinguishers are pressurized with air to approximately 150 pounds per square inch (psi)—five times a car tire pressure—from a compressor. A squeeze-grip handle operates a spring-loaded valve threaded into the pressure cylinder. Inside, a pipe or "dip tube" extends to the bottom of the tank so that in the upright position, the opening of the tube is submerged. The water is released as a steady stream through a hose or nozzle, pushed out by the stored pressure above it.
Water extinguishers of the "gas cartridge" type operate in much the same manner, but the pressure source is a small cartridge of carbon dioxide gas (CO 2 ) at 2,000 psi, rather than air. To operate a gas cartridge unit, the end of the extinguisher is struck against the floor, causing a pointed spike to pierce the cartridge, releasing the gas into the pressure vessel. The released CO 2 expands several hundred times its original volume, filling the gas space above the water. This pressurizes the cylinder and forces the water up through a dip-pipe and out through a hose or nozzle to be directed upon the fire. This design proved to be less prone to leakdown (loss of pressure over time) than simply pressurizing the entire cylinder.
In foam extinguishers, the chemical agent is generally held under stored pressure. In dry powder extinguishers, the chemicals can either be put under stored pressure, or a gas cartridge expeller can be used; the stored-pressure type is more widely used. In carbon dioxide extinguishers, the CO 2 is retained in liquid form under 800 to 900 psi and is "self-expelling," meaning that no other element is needed to force the CO 2 out of the extinguisher. In halon units, the chemical is also retained in liquid form under pressure, but a gas booster (usually nitrogen) is generally added to the vessel.
Raw Materials
Fire extinguishers can be divided into four classifications: Class A, Class B, Class C, and Class D. Each class corresponds to the type of fire the extinguisher is designed for, and, thus, the type of extinguishing agents used. Class A extinguishers are designed to fight wood and paper fires; Class B units fight contained flammable liquid fires; Class C extinguishers are designed to fight live electrical fires; and Class D units fight burning metal fires.
Water has proven effective in extinguishers used against wood or paper fires (Class A). Water, however, is an electrical conductor. Naturally, for this reason, it is not safe as an agent to fight electrical fires where live circuits are present (Class C). In addition, Class A extinguishers should not to be used in the event of flammable liquid fires (Class B), especially in tanks or vessels. Water can cause an explosion due to flammable liquids floating on the water and continuing to burn. Also, the forceful water stream can further splatter the burning liquid to other combustibles. One disadvantage of water extinguishers is that the water often freezes inside the extinguisher at lower temperatures. For these reasons, foam, dry chemical, CO 2 , and halon types were developed.
Foam, although water based, is effective against fires involving contained flammable liquids (Class B). A two-gallon (7.5 liters) extinguisher will produce about 16 gallons (60 liters) of thick, clinging foam that cools and smothers the fire. The agent itself is a proprietary compound developed by the various manufacturers and contains a small amount of propylene glycol to prevent freezing. It is contained as a mixture in a pressurized cylinder similar to the water type. Most aircraft carry this type of extinguisher. Foam can also be used on Class A fires.
The dry powder agent was developed to reduce the electrical hazard of water, and thus is effective against Class C fires. (It can also be used against Class B fires.) The powder is finely divided sodium bicarbonate that is extremely free-flowing. This extinguisher, also equipped with a dip-tube and containing a pressurizing gas, can be either cartridge-operated or of the stored pressure type as discussed above. Many specialized dry chemical extinguishers are also suitable for burning metal fires, or Class D.
Carbon dioxide (CO 2 ) extinguishers, effective against many flammable liquid and electrical fires (Class B and C), use CO 2 as both the agent and the pressurizing gas. The liquified carbon dioxide, at a pressure that may exceed 800 psi depending on size and use, is expelled through a flared horn. Activating the squeeze-grip handle releases the CO 2 into the air, where it immediately forms a white, fluffy "snow." The snow, along with the gas, substantially reduces the amount of oxygen in a small area around the fire. This suffocates the fire, while the snow clings to the fuel, cooling it below the combustion point. The greatest advantage to the CO 2 extinguisher is the lack of permanent residue. The electrical apparatus that was on fire is then more likely to be able to be repaired. Unlike CO 2 "snow," water, foam, and dry chemicals can ruin otherwise undamaged components.
As extinguishing agents, halons are up to ten times more effective in putting out fires than other chemicals. Most halons are non-toxic and extremely fast and effective. Chemically inert, they are harmless to delicate equipment, including computer circuits, and leave no residue. The advantage of the halon over the CO 2 extinguisher is that it is generally smaller and lighter. Halon is a liquid when under pressure, so it uses a dip-tube along with nitrogen as the pressurizing gas.
Halon, at least in fire extinguishers, may soon become a footnote to history. In 1992, 87 nations around the world agreed to halt the
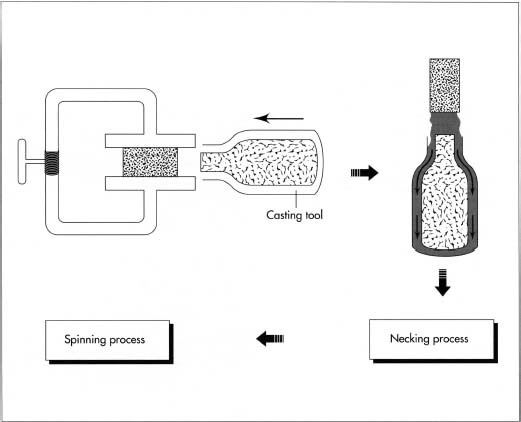
This cylinder is then finished in necking and spinning processes, which form the open end of the cylinder.
Most of the other elements of a fire extinguisher are made of metal. The pressure vessel is generally made of an aluminum alloy, while the valve can either be steel or plastic. Other components, such as the actuating handle, safety pins, and mounting bracket, are typically made of steel.
The Manufacturing
Process
Manufacture of the tank-type or cylinder fire extinguisher requires several manufacturing operations to form the pressure vessel, load the chemical agent, machine the valve, and add the hardware, hose, or nozzle.
Creating the pressure vessel
- 1 Pressure vessels are formed from puck-shaped (disc) blocks of special aluminum alloy. The puck is first impact extruded on a large press under great pressure. In impact extrusion, the aluminum block is put into a die and rammed at very high velocity with a metal tool. This tremendous energy liquifies the aluminum and causes it to flow into a cavity around the tool. The aluminum thus takes the form of an open-ended cylinder with considerably more volume than the original puck.
Necking and spinning
-
2 The necking process puts a dome on the open end of the cylinder by
constricting
the open end with another operation called spinning. Spinning gently rolls the metal together, increasing the wall thickness and reducing the diameter. After spinning, the threads are added.
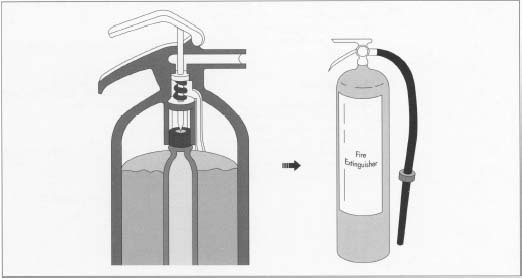 In a typical gas-cartridge extinguisher, a spike pierces the gas cartridge. The released gas expands quickly to fill the space above the water and pressurize the vessel. The water can then be pumped out of the extinguisher with the necessary force.
In a typical gas-cartridge extinguisher, a spike pierces the gas cartridge. The released gas expands quickly to fill the space above the water and pressurize the vessel. The water can then be pumped out of the extinguisher with the necessary force. - 3 The vessel is hydrostatically tested, cleaned, and coated with a powdered paint. The vessel is then baked in an oven where the paint is cured.
Adding the extinguishing agent
- 4 Next, the extinguishing agent is added. If the vessel is a "stored-pressure" type, the vessel is then pressurized accordingly. If a gas-cartridge is necessary to help expel the extinguishing agent, it is also inserted at this time.
- 5 After the extinguishing element is added, the vessel is sealed and the valve is added. The valve consists of a machined body made of metal bar stock on a lathe, or a plastic injected molded part on the economy versions. It must be leak free, and it must have provisions for threading into the cylinder.
Final assembly
- 6 The final manufacturing operation is the assembly of the actuating handle, safety pins, and the mounting bracket. These parts are usually cold formed—formed at low temperatures—steel or sheet metal forms, purchased by the manufacturer from an outside vendor. Identification decals are also placed on the cylinder to identify the proper fire class rating as well as the suitability for recharging. Many of the economy versions are for one time use only and cannot be refilled.
Quality Control
All fire extinguishers in the United States fall under the jurisdiction of the National Fire Protection Association (NFPA), Under-writer's Laboratories, The Coast Guard, and other organizations such as the New York Fire Department. Manufacturers must register their design and submit samples for evaluation before marketing an approved fire extinguisher.
One of the most crucial checkpoints during the manufacturing process occurs after the extinguishing agent is added and the vessel sealed. It is extremely important that the cylinder not leak down the pressurizing gas, because that would render the extinguisher useless. To check for leaks, a boot is placed over the cylinder to serve as an accumulator. A trace gas is released inside, and within two minutes any unacceptable rate of leakage can be recorded by sophisticated pressure and gas-detecting equipment. All extinguishers are leak tested.
The Future
With the gradual elimination of halon, a new, non-damaging agent will most likely replace the hazardous chemical within the next few years. In addition, new applications of the old designs are being seen; most prevalent are automatic heat and fire sensors that discharge the extinguisher without the need for an operator.
Where To Learn More
Books
Fire Prevention Handbook. Butterworths, London, 1986.
Mahoney, Gene. Introduction to Fire Apparatus & Equipment. 2nd ed., Fire Engineering Books & Videos, 1986.
Pamphlets
Portable Fire Extinguishing Equipment in Family Dwellings & Living Units. National Fire Protection Association, 1992.
— Douglas E. Betts and
Peter Toeg
Its 9" long 8" diameter and weighs around 4 lbs. It is 90% Carbon Tetrachloride
Thanks! Kay
What is the actual process of making/manufacturing Fire extinguisher ?
What are the ingredients/contents used in the manufacturing process ?
Please mail me the answers if possible.
Thank You