Laundry Detergent
Background
The first soaps were manufactured in ancient times through a variety of methods, most commonly by boiling fats and ashes. Archeologists excavating sites in ancient Babylon have found evidence indicating that such soaps were used as far back as 2800 B.C. By the second century A.D., the Romans were regularly making soap, which they had probably begun to produce even earlier.
In Europe, the use of soap declined during the Middle Ages. However, by the fifteenth century, its use and manufacture had resumed, and an olive-oil based soap produced in Castile, Spain, was being sold in many parts of the known world. Castile soap, which is still available today, has retained its reputation as a high-quality product.
During the colonial period and the eighteenth century, Americans made their own soap at home, where most continued to produce it until soap manufacture shifted away from individual homes to become an industry during the 1930s. The first detergent, or artificial soap, was produced in Germany during World War I. In 1946, the first built detergent appeared, comprising a surfactant (a surface-acting agent or soap) and a builder (a chemical that enhances the performance of the surfactant as well as rendering the laundering process more effective in other ways). Pushed along by economic prosperity and the development of relatively inexpensive washing machines in the wake of World War II, detergent sales soared; by 1953, they had surpassed soap sales in the United States.
Raw Materials
Although people commonly refer to laundry detergent as "soap," it is actually a synthetic combination that functions much like soap, with certain major improvements. Soap cleans because each soap molecule consists of a hydrocarbon chain and a carboxylic group (fatty acids) that perform two important functions. The carboxylate end of the soap molecule is hydrophilic, meaning that it is attracted to water, while the hydrocarbon end of the molecule is both hydrophobic (repelled by water) and attracted to the oil and grease in dirt. While the hydrophobic end of a soap molecule attaches itself to dirt, the hydrophilic end attaches itself to water. The dirt attached to the carboxylate end of the molecule is chemically dragged away from the clothes being cleaned and into the wash water. Properly agitating and rinsing the clothes furthers the cleansing process.
The major difficulty with using soap to clean laundry shows up when it is used in hard water—water that is rich in natural minerals such as calcium, magnesium, iron, and manganese. When these chemicals react with soap, they form an insoluble curd called a precipitate. Difficult to rinse out, the precipitate leaves visible deposits on clothing and makes fabric feel stiff. Even water that is not especially hard will eventually produce precipitates over a period of time.
While the hydrocarbons used in soap generally come from plants or animals, those used in detergent can be derived from crude oil. Adding sulfuric acid to the processed hydrocarbon produces a molecule similar to the fatty acids in soap. The addition of an alkali to the mixture creates a surfactant molecule
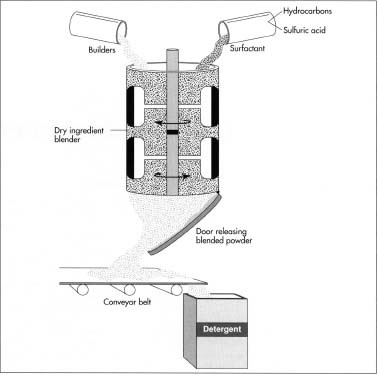
In addition to a surfactant, modern detergent contains several other ingredients. Among the most significant are builders, chemicals which serve several purposes. Most importantly, they increase the efficiency of the surfactant. They also sequester minerals in hard water, meaning that they hold them in solution, preventing them from precipitating out. Furthermore, builders can emulsify oil and grease into tiny globules that can be washed away. Some, like sodium silicate, inhibit corrosion and help assure that the detergent will not damage a washing machine. Still other builders contribute to the chemical balance of the wash water, making sure that it conduces to effective washing.
Modern detergents have several other ingredients including antiredeposition agents, chemicals that help prevent soil from settling back on washed clothes. Fluorescent whitening agents are also common. By converting invisible ultraviolet light into visible blue light, these help to maintain brightness or whiteness. Oxygen bleaches such as sodium perborate improve the detergency of the mixture, especially in low-phosphate or no-phosphate products, as well as helping to remove some types of stains. Processing aids such as sodium sulfate are also used to prevent caking and to standardize product density.
Enzymes and perfumes are also found in commercial detergents. Enzymes (a type of protein) break down some stains to make them easier to remove and are an essential ingredient in various pre-soak products used to treat heavily soiled clothes prior to laundering. Perfumes or fragrances cover the odor of the dirt and any chemical smell from the detergent itself. Suds control agents also have a role in detergents—too many suds can cause mechanical problems with a washing machine.
The Manufacturing
Process
Although there are three ways of manufacturing dry laundry detergent, only two are commonly used today. In the blender process favored by smaller companies, the ingredients are mixed in large vats before being packaged. The machines used are very large: a common blender holds 4,000 pounds (1,816 kilograms) of mixed material, but the blenders can accommodate loads ranging from 500 to 10,000 pounds (227 to 4,540 kilograms). By industry standards, these are small batches for which the blender process is ideal. While some settling may occur, the resulting detergent is of high quality and can compete with detergents made by other processes. The second commonly used method of production is called the agglomeration process. Unlike the blender process, it is continuous, which makes it the choice of very large detergent manufacturers. The agglomeration process can produce between 15,000 and 50,000 pounds (6,800 and 22,700 kilograms) of detergent per hour. In the third method, dry ingredients are blended in water before being dried with hot air. Although the resulting product is of high quality, the fuel costs and engineering problems associated with venting, reheating, and reusing the air have led to this method being largely replaced by agglomeration.
The blender process
- 1 First, ingredients are loaded into one of two machines: a tumbling blender or a ribbon blender. The tumbling blender, shaped like a rectangular box, is turned and shaken from outside by a machine, while the ribbon blender is a cylinder fitted with blades to scrape and mix the ingredients. After the ingredients inside the blender have been mixed, a doorway at the bottom of the bowl is opened. With the blender still agitating the ingredients, the mix is allowed to run out onto a conveyor belt or other channeling device. The belt then moves the detergent to another area of the factory where it can be dropped into boxes or cartons for delivery to wholesalers or distributors.
The agglomeration process
- 2 In this method, dry ingredients for a detergent are first fed into a large machine known as a Shuggi agglomerator (Shuggi is the manufacturer). Inside the agglomerator, sharp, whirling blades mix the material to a fine consistency; the process resembles food being textured inside a food processor.
- 3 After the dry ingredients have been blended, liquid ingredients are sprayed on the dry mix through nozzles fitted into the agglomerator's walls. The blending continues, causing an exothermic (heat-producing) reaction to occur. The resulting mixture is a hot, viscous liquid similar to gelatin that hasn't hardened.
- 4 Next, the liquid is allowed to flow out of the agglomerator. As it leaves the machine, it collects on a drying belt where its own heat, exposure to air, and hot air blowers render it friable—easy to crush or crumble. The newly made detergent is then pulverized and pushed through sizing screens that ensure that no large lumps of unmixed product go out to the market. The result of this process is a dry detergent made up of granules of the mixed detergent.
The slurry method
- 5 In this process, ingredients are dissolved in water to create a slurry. With a pump, the slurry is blown through nozzles inside the top of a cone shaped container as hot, dry air is simultaneously forced into the bottom of the cone. As the slurry dries, "beads" of dry detergent fall to the bottom of the cone, where they can be collected for packaging.
Liquid detergent
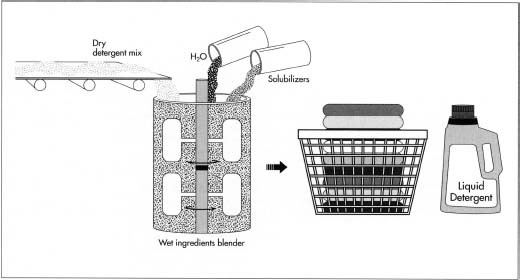
- 6 If the detergent is to be liquid rather than powder, it is simply mixed back in—after all ingredients are blended—with a solution consisting of water and various chemicals known as solubilizers. The solubilizers help the water and detergent blend together more fully and evenly.
Quality Control
Manufacturers constantly monitor the quality of their detergents, and they utilize the same testing methods to assess the effectiveness of new products. In one method, light is shined onto a piece of fabric that has been soiled and then washed in the test detergent. The
Another method involves laboratory burning of a small amount of material that has been soiled and then laundered. The weight of the ashes, plus the weight of the gaseous results of the burning, reveal how much of the dirt remained in the fabric after laundering. A result that is much higher than a clean test sample indicates that a significant amount of dirt was retained in the laundered sample. Naturally, the goal is to come as close to the weight of a clean control sample as possible.
Byproducts
In recent years, the laundry detergent industry has been faced with two environmental challenges, both of which have seem to have been dealt with successfully. Environmentalists were concerned that phosphate builders added large amounts of phosphorous compounds to the nation's waterways. Acting as a fertilizer, the phosphorus stimulated the growth of algae, and these unnaturally large crops of algae significantly depleted the amount of dissolved oxygen in water. This decrease in free oxygen harmed other marine life, thus threatening to disrupt normal ecological patterns.
This problem, and the environmental pressure and legislation it prompted in the late 1960s, led manufacturers to develop effective builders that did not contain phosphates. Today, detergents sold in many states are phosphate-free. Although this adjustment did not entail a change in the manufacturing process, it did require a research effort that took several months to devise a satisfactory alternative.
An earlier environmental problem was that of excess detergent foam appearing in the nation's waterways. In the early 1950s, when home use of washing machines and laundry detergents grew at an explosive rate, there were several instances of large amounts of foam appearing in rivers and streams, although detergent may not have been the only cause of the foaming. Over a period of five years, from 1951 to 1956, it was found that a common surfactant, ABS (alkyl benzene sulfonate), the detergent ingredient that contributed to foaming, was responsible. ABS's complex molecular structure did not biodegrade rapidly enough to keep it from foaming once washing water was discharged. A proven replacement was not immediately available. Beginning in 1956, however, manufacturers replaced ABS with LAS (linear alkylate sulfonate), which biodegrades rapidly, and since that time, LAS has been the primary foaming agent in detergents.
Where To Learn More
Books
De Groot, W. Herman. Sulphonation Technology in the Detergent Industry. Kluwer Academic Publishers, 1991.
Periodicals and Pamphlets
Marbach, William D. "Cleaner Clothes from Worms." Business Week. December 7, 1992, p. 123.
Pinder, Jeanne B. "Laundry Detergent Takes Formula from Nature." New York Times. March 10, 1993, p. C3 (N).
Smith, Emily T. "Fungus around the Collar Could Clean Up Lipstick Stains." Business Week. February 15, 1988, p. 107.
Soaps and Detergents. The Soap and Detergent Association, 1981.
— Lawrence H. Berlow
I would like to have a more detailed write up on Agglomeration method to understand the large volume mfg.process and Quality control parameters.
With best regards
Ajay
with best regards
and also make enquire about the equipment.