Lubricating Oil
Background
Since the Roman era, many liquids, including water, have been used as lubricants to minimize the friction, heat, and wear between mechanical parts in contact with each other. Today, lubricating oil, or lube oil, is the most commonly used lubricant because of its wide range of possible applications. The two basic categories of lube oil are mineral and synthetic. Mineral oils are refined from naturally occurring petroleum, or crude oil. Synthetic oils are manufactured polyalphaolefins, which are hydrocarbon-based polyglycols or ester oils.
Although there are many types of lube oils to choose from, mineral oils are the most commonly used because the supply of crude oil has rendered them inexpensive; moreover, a large body of data on their properties and use already exists. Another advantage of mineral-based lube oils is that they can be produced in a wide range of viscosities—viscosity refers to the substance's resistance to flow—for diverse applications. They range from low-viscosity oils, which consist of hydrogen-carbon chains with molecular weights of around 200 atomic mass units (amu), to highly viscous lubricants with molecular weights as high as 1000 amu. Mineral-based oils with different viscosities can even be blended together to improve their performance in a given application. The common 1OW-30 motor oil, for example, is a blend of low viscous oil (for easy starting at low temperatures) and highly viscous oil (for better motor protection at normal running temperatures).
First used in the aerospace industry, synthetic lubricants are usually formulated for a specific application to which mineral oils are ill-suited. For example, synthetics are used where extremely high operating temperatures are encountered or where the lube oil must be fire resistant. This article will focus on mineral-based lube oil.
Raw Materials
Lube oils are just one of many fractions, or components, that can be derived from raw petroleum, which emerges from an oil well as a yellow-to-black, flammable, liquid mixture of thousands of hydrocarbons (organic compounds containing only carbon and hydrogen atoms, these occur in all fossil fuels). Petroleum deposits were formed by the decomposition of tiny plants and animals that lived about 400 million years ago. Due to climatic and geographical changes occurring at that time in the Earth's history, the breakdown of these organisms varied from region to region.
Because of the different rates at which organic material decomposed in various places, the nature and percentage of the resulting hydrocarbons vary widely. Consequently, so do the physical and chemical characteristics of the crude oils extracted from different sites. For example, while California crude has a specific gravity of 0.92 grams/milliliter, the lighter Pennsylvania crude has a specific gravity of 0.81 grams/milliliter. (Specific gravity, which refers to the ratio of a substance's weight to that of an equal volume of water, is an important aspect of crude oil.) Overall, the specific gravity of crudes ranges between 0.80 and 0.97 grams/milliliter.
Depending on the application, chemicals called additives may be mixed with the
The Manufacturing
Process
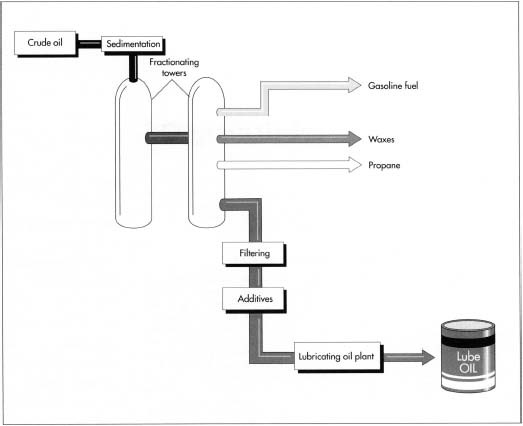
Lube oil is extracted from crude oil, which undergoes a preliminary purification process (sedimentation) before it is pumped into fractionating towers. A typical high-efficiency fractionating tower, 25 to 35 feet (7.6 to 10.6 meters) in diameter and up to 400 feet (122 meters) tall, is constructed of high grade steels to resist the corrosive compounds present in crude oils; inside, it is fitted with an ascending series of condensate collecting trays. Within a tower, the thousands of hydrocarbons in crude oil are separated from each other by a process called fractional distillation. As the vapors rise up through the tower, the various fractions cool, condense, and return to liquid form at different rates determined by their respective boiling points (the lower the boiling point of the fraction, the higher it rises before condensing). Natural gas reaches its boiling point first, followed by gasoline, kerosene, fuel oil, lubricants, and tars.
Sedimentation
- 1 The crude oil is transported from the oil well to the refinery by pipeline or tanker ship. At the refinery, the oil undergoes sedimentation to remove any water and solid contaminants, such as sand and rock, that maybe suspended in it. During this process, the crude is pumped into large holding tanks, where the water and oil are allowed to separate and the contaminants settle out of the oil.
Fractionating
- 2 Next, the crude oil is heated to about 700 degrees Fahrenheit (371 degrees Celsius). At this temperature it breaks down into a mixture of hot vapor and liquid that is then pumped into the bottom of the first of two fractionating towers. Here, the hot hydrocarbon vapors float upward. As they cool, they condense and are collected in different trays installed at different levels in the tower. In this tower, normal atmospheric pressure is maintained continuously, and about 80 percent of the crude oil vaporizes.
- 3 The remaining 20 percent of the oil is then reheated and pumped into a second tower, wherein vacuum pressure lowers the residual oil's boiling point so that it can be made to vaporize at a lower temperature. The heavier compounds with higher boiling points, such as tar and the inorganic compounds, remain behind for further processing.
Filtering and solvent extraction
- 4 After further processing to remove unwanted compounds, the lube oil that has been collected in the two fractionating towers is passed through several ultrafine filters, which remove remaining impurities. Aromatics, one such contaminant, contain six-carbon rings that would affect the lube oil's viscosity if they weren't removed in a process called solvent extraction. Solvent extraction is possible because aromatics are more soluble in the solvent than the lube oil fraction is. When the lube oil is treated with the solvent, the aromatics dissolve; later, after the solvent has been removed, the aromatics can be recovered from it.
Additives, inspection, and packaging
- 5 Finally, the oil is mixed with additives to give it the desired physical properties (such as the ability to withstand low temperatures). At this point, the lube oil is subjected to a variety of quality control tests that assess its viscosity, specific gravity, color, flash, and fire points. Oil that meets quality standards is then packaged for sale and distribution.
Quality Control
Most applications of lube oils require that they be nonresinous, pale-colored, odorless, and oxidation-resistant. Over a dozen physical and chemical tests are used to classify and determine the grade of lubricating oils. Common physical tests include measurements for viscosity, specific gravity, and color, while typical chemical tests include those for flash and fire points.
Of all the properties, viscosity, a lube oil's resistance to flow at specific temperatures and pressures, is probably the single most important one. The application and operating temperature range are key factors in determining the proper viscosity for an oil. For example, if the oil is too viscous, it offers too much resistance to the metal parts moving against each other. On the other hand, if it not viscous enough, it will be squeezed out from between the mating surfaces and will not be able to lubricate them sufficiently. The Saybolt Standard Universal Viscometer is the standard instrument for determining viscosity of petroleum lubricants between 70 and 210 degrees Fahrenheit (21 and 99 degrees Celsius). Viscosity is measured in the Say bolt Universal second, which is the time in seconds required for 50 milliliters of oil to empty out of a Saybolt viscometer cup through a calibrated tube orifice at a given temperature.
The specific gravity of an oil depends on the refining method and the types of additives present, such as lead, which gives the lube oil the ability to resist extreme mating surface pressure and cold temperatures. The lube oil's color indicates the uniformity of a particular grade or brand. The oil's flash and fire points vary with the crude oil's origin. The flash point is the temperature to which an oil has to be heated until sufficient flammable vapor is driven off so that it will flash when brought into contact with a flame. The fire point is the higher temperature at which the oil vapor will continue to burn when ignited.
Common engine oils are classified by viscosity and performance according to specifications established by the Society of Automotive Engineers (SAE). Performance factors include wear prevention, oil sludge deposit formation, and oil thickening.
The Future
The future of mineral-based lubricating oil is limited, because the natural supplies of petroleum are both finite and non-renewable. Experts estimate the total recoverable light to medium petroleum reserves at 1.6 trillion barrels, of which a third has been used. Thus, synthetic-based oils will probably be increasingly important as natural reserves dwindle. This is true not only for lubricating oil but also for the other products that result from petroleum refining.
Where To Learn More
Books
Fuels, Lubricants, and Coolants, 7th ed. Deere & Company Service Publications, 1992.
Malone, L. J. Basic Concepts of Chemistry. John Wiley & Sons, Inc., 1989.
Nadkarni, R. A., ed. Analysis of Petroleum Products & Lubricants. American Society for Testing & Materials, 1991.
Seal, Shirley C., ed. Fluids, Lubricants & Sealing Devices. National Fluid Power Association, 1989.
Periodicals
Bienkowski, Keith. "Coolants and Lubricants: The Truth." Manufacturing Engineering. March, 1993.
"System Provides Real-Time Lube Oil Blending." Design News. February 26, 1990, p. 39.
O'Lenick, Anthony and Raymond E. Bilbo. "Saturated Liquid Lubricant Withstands Aluminum Forming." Research & Development. February, 1989, p. 162.
Peterson, Ivars. "Friction Features." Science News. April 30, 1988, p. 283.
Templeton, Fleur. "The Right Lube Job for Superhot Ceramic Engines?" Business Week. May 18, 1992, p. 113.
Vogel, Todd, John Rossant, and Sarah Miller. "Oil's Rude Awakening." Business Week. September 26, 1988, p. 44.
— Craig F. Whitlow
(2)no line of lubricating oil is shown from the atms. disillation?so my questions are:
1- the products of an atmo.distillation column from the top to the bottom are,Gas,total naphtha, kerosine, LGO,HGO and residue. can you tel me from which slot we pull the lubricating oil?
2- the product of Vac. distillation column from top to bottom are: wilde distillate VLGO.VHGO and bunker fuel. can you tell me from which slot we pull lubricating oil. thank you in advance
Want to have details on it.