Fireworks
Background
A firework is a device that uses combustion or explosion to produce a visual or auditory effect. Modern pyrotechnics also includes devices similar to fireworks, such as flares, matches, and even solid-fuel rocket boosters used in spaceflight.
The earliest ancestors of fireworks were paper or bamboo tubes filled with finely grourid charcoal and sulfur used in China two thousand years ago. These tubes produced a flash of fire and smoke when ignited, but no explosion. True fireworks did not exist until saltpeter was added to the mixture to create black powder, the first chemical explosive, one thousand years later. Black powder was probably first made in China, but some scholars suggest that it may have been invented by the Arabs.
The Chinese used black powder for fire-works, signals, and weapons such as bombs and rockets. Black powder was introduced to Europe in the 14th century as an explosive for both fireworks and guns. It was applied to mining and roadbuilding projects by the late 17th century. Black powder was used for gunpowder until it was replaced by nitrocellulose in the late 19th century, and (for industrial purposes) by dynamite in the early 20th century, but it is still used in fire-works today.
Fireworks in China evolved from simple firecrackers to the extravagant displays witnessed by European explorers in the 16th century. In Europe, fireworks began with military explosives adapted for use in celebrating victories and progressed to the elaborate productions designed by Italian pyrotechnists in the 16th, 17th, and 18th centuries. (Even today, most of the large fire-work companies in the United States are run by Italian-American families.) These Italian fireworks were usually shown on lavishly decorated wooden sets, often floating on bodies of water, both for safety and to reflect the beautiful displays. On the other hand, German fireworks of the time were usually shot into the air, much like today's fireworks.
Although the firework displays of the Italian masters were extremely complex and impressive works of art, the technology of the time limited their color and brightness. During the 19th century, the introduction of aluminum and magnesium greatly increased the brightness, while the development of potassium chlorate by the French chemist Claude-Louis Berthollet (who was trying to improve the gunpowder used by Napoleon's troops) made it possible to produce more intense colors.
Fireworks came to the New World with the earliest settlers, and have been used to celebrate Independence Day, July 4, since the earliest days of the United States. During the early 20th century these fireworks became bigger, more powerful, and dangerous. Between 1900 and 1930 more than 4,000 people were killed by fireworks. Federal and state governments began regulating the use of fireworks in the 1930s. Explosives are classified as Class A (dangerous substances such as dynamite and TNT), Class B (fireworks used for professional displays) and Class C (smaller fireworks intended for private use.) Class C fireworks must not contain more than 50 milligrams of explosive. Some states allow all Class C
While the private use of fireworks is heavily restricted, public displays have become more and more elaborate. Computers are used to time fireworks precisely, so they can be choreographed in time to music. Lasers are sometimes used to produce unique visual effects. Today fireworks are made and displayed around the world, particularly in Europe, Latin America, the United States, and Japan.
Raw Materials
A modern firework consists of a shell of plastic, papier-mache, or heavy paper surrounding compartments separated by cardboard. A small compartment at the base of the shell contains black powder to propel the firework into the sky from a mortar made of iron, aluminum, plastic, or heavy cardboard. A larger compartment contains chunks of a mixture of chemicals that produce light and color when heated. These chunks are known as stars. In American and European fireworks the stars are mixed with black powder inside a cylindrical compartment. The black powder explodes to ignite the stars and scatter them across the sky. In Asian fireworks the stars surround the black powder in a spherical compartment to produce a more symmetrical display. Instead of black powder and stars a compartment may contain flash powder, which produces a sudden bright light and loud bang. The various compartments in a firework are attached to fuses made of threads mixed with grains of gunpowder.
Black powder consists of a mixture of salt-peter (potassium nitrate), charcoal, and sulfur in a 75 to 15 to 10 ratio by weight. Flash powder consists of a mixture of potassium chlorate or potassium perchlorate, sulfur, and aluminum. Stars consist of a fuel that burns to provide heat, a coloring agent that provides color when heated, and an oxidizer to burn the fuel. Fuels may be slow-burning such as charcoal, dextrin (derived from corn starch), or red gum (a tree secretion) to produce a dim, long-lasting display, or fast-burning, such as aluminum, magnesium, or titanium, to produce a bright, short-lasting display. Sugar may be used as a fuel to produce smoke. Coloring agents include aluminum, magnesium, or titanium (white), carbon or iron (orange), sodium compounds (yellow), copper compounds (blue), strontium carbonate (red), and barium nitrate or barium chlorate (green). Oxidizers are highly reactive oxygen-containing compounds such as potassium perchlorate or ammonium perchlorate. They also contain chlorine, which reacts with the copper, strontium, and barium compounds in the
The Manufacturing
Process
Making the stars
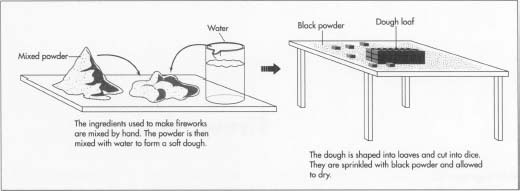
- 1 The ingredients used to prepare stars are obtained from chemical supply companies and stored in barrels. At the time of mixing the chemicals are scooped out of the barrels, weighed, and sifted twice through brass screens to remove lumps. (Brass is used because it does not produce sparks.) The sifted powders are placed on a large sheet of paper and gently mixed by hand. The powders may also be mixed inside a rotating drum or a stationary container with rotating paddles. These devices must be used with great care to avoid generating heat through friction or trapping bits of powder between moving parts.
- 2 The mixed powder is placed in barrels and taken from the mixing room to the cutting room. Water is mixed with it to form a soft dough. Lumps of the dough are scooped into large paper-lined wooden molds shaped like loaves of bread. The dough is packed firmly into the mold with a wooden mallet. (The wet dough is much safer to manipulate than the dry powder.)
- 3 The loaves of dough, weighing about 35 pounds (16 kg) each, are unmolded onto a workbench covered with heavy cardboard sprinkled with black powder. The loaves are cut in one direction to form slices then cut in the other direction to form dice. The dimensions of the dice may be anywhere from 0.06-2 inches (0.16-5 cm). The black powder adheres to the wet dice and will help them burn when the firework is ignited. The dice are allowed to dry on papercovered screens.
Making the breaks
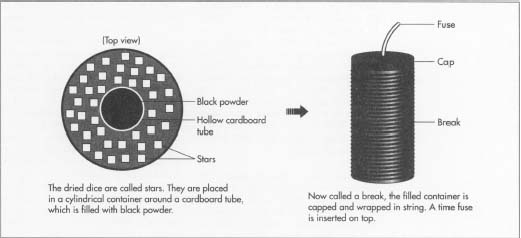
- 4 The dried dice are now stars. They are moved to the packing room to be placed into cardboard containers. A hollow cardboard tube is placed in the center of the cylindrical container and stars are gently poured around it. A large container may hold as many as 900 stars (about 4.4 pounds [2 kg]). When the container is full, black powder is poured inside the hollow tube and the tube is removed. The powder fills the spaces between the stars, and will serve to ignite and scatter them. A paper cap is placed on the filled container, now called a "break."
- 5 The break is wrapped with heavy string, a process known as spiking. Spiking is done by tying one end of a large spool of string to the break and winding the string around it. When the break is completely covered, the string is cut and tied. Some breaks are not spiked, but are made instead of plastic or heavier cardboard to withstand the stress of being launched. A time fuse (a short, slow-burning fuse that causes the break to explode a certain amount of time after it is launched) is inserted into the break, and it is wrapped in heavy paper. The wrapped breaks are moved to the pasting room to be wrapped in heavy, paste-soaked paper, then allowed to dry for about two days. The paper hardens as the paste dries to form a strong, tight seal.
- 6 Some breaks, known as salutes, are filled with flash powder rather than stars and black powder. Flash powder is mixed in much the same way as the chemicals used to make stars. It is then poured into cardboard containers that are thicker and stronger than other breaks. This allows more pressure to build up before the salute bursts, resulting in a louder bang. These salutes are then spiked and pasted like other breaks.
Making the shells
- 7 The dry breaks are moved to the finishing room to be assembled into shells. The simplest shells consist of a small compartment of black powder combined with a single break. Due to their spherical structure, Asian shells always contain only one break. Because American and European shells are cylindrical, more than one break can be stacked together, so that the shell will display multiple bursts of different colors when it explodes. Multi-break shells usually consist of a small compartment of black powder, three or four colored breaks, and a salute. Some large shells contain as many as 10 breaks, and at least one gigantic shell has been made holding 22 breaks. The shell is assembled by stacking the components together, attaching a starting fuse (a long, fast-burning fuse used to ignite the black powder that launches the firework), wrapping them in heavy paper, and tying the package together with string. The completed firework is then labeled and stored until needed.
Making small fireworks
- 8 Small fireworks, intended for private use, are made in much the same way as large ones, but they are generally simpler in construction and contain much less explosive. Small fireworks include firecrackers (paper tubes holding a small amount of explosive), fountains (paper cones filled with chemicals which release colored sparks), and Roman candles (long paper tubes filled with a small amount of explosive and several small stars which shoot out one at a time). Some small fireworks contain no explosive at all and may be as simple as a single chemical wrapped in paper or foil. Examples include smoke balls (filled with a chemical that releases colored smoke) and snakes (filled with ammonium dichromate, which slowly burns and produces a long trail of ash). Sparklers are made by dipping a metal wire in a slurry containing a fuel, an oxidizer, a coloring agent, and aluminum granules, which provide the sparks.
Launching the fireworks
- 9 Professional fireworks are usually launched by the same companies who make them. If a set piece (a ground-based display that forms a picture or words with colored flares called lances) is to be used, the design to be formed is sketched on graph paper and sent to carpenters who build a wooden frame with thin wooden slats in the shape of the design. If music will accompany the fireworks, the timing of the display is planned to match the tempo of the music.
- 10 Several hours before the show begins (or a few days in advance, for a very large show), the crew arrives with all the necessary equipment, including fire extinguishers and first aid kits. Mortars to launch the shells are placed in their proper places. Large ones are placed in holes dug in the ground or in steel drums filled with sand. Smaller mortars are placed in wooden racks. The proper shell for each mortar is loaded in place. The frames for set pieces are assembled, lances are attached to the slats, and fuses are attached to the lances. When the display begins, the lances and mortars are lit at the proper times, either with long hand-held flares or with electrical wires attached to a central switchboard. After the show, the crew safely destroys any unexploded duds.
Quality Control
The most important quality control factor in making fireworks is safety. Firework factories are protected from intruders by chain-link fences, barbed wire, locked gates, steel doors, and tamper-proof locks. Within these factories, numerous precautions are taken to prevent accidents.
Electricity is the greatest danger. A single small spark can set off a roomful of explosives. All electrical outlets are located out-side the building. To avoid generating static electricity, all workers must wear 100% cotton clothing. They touch a copper plate before they enter a building to remove any static electricity they may be carrying. Elastic straps with wires trailing to the graphite floor are worn around the worker's calves, to drain static electricity away to grounding rods buried beneath the building. All work is halted and all workers leave the building if there is any possibility of an electrical storm approaching.
Many other safety measures are used. All work is done by hand, to avoid machines that could produce heat or sparks. In the winter, buildings are heated with hot water rather than hot air, which could cause an explosion. The buildings are small, so no one is more than one or two steps away from an exit. All exits have doors that open wide at the slightest touch. Explosive chemicals are never mixed when wet, because when they dry out they may release gases that could ignite them.
Where To Learn More
Books
Brenner, Martha. Fireworks Tonight! Hastings House, 1986.
Plimpton, George. Fireworks. Doubleday, 1984.
Periodicals
Begley, Sharon. "Up in the Sky! It's…Hearts! Stars! Bow Ties!" Newsweek, July 9, 1990, p. 60.
Conkling, John A. "Pyrotechnics." Scientific American, July 1990, pp. 96-102.
Kozlou, Alex. "First Family of Fireworks." Discover, July 1990, pp. 40-45.
— Rose Secrest