Artificial Skin
Background
Skin, the human body's largest organ, protects the body from disease and physical damage, and helps to regulate body temperature. It is composed of two major layers, the epidermis and the dermis. The epidermis, or outer, layer is composed primarily of cells: keratinocytes, melanocytes, and langerhans. The dermis, composed primarily of connective tissue fibers such as collagen, supplies nourishment to the epidermis.
When the skin has been seriously damaged through disease or burns, the body cannot act fast enough to manufacture the necessary replacement cells. Wounds, such as skin ulcers suffered by diabetics, may not heal and limbs must be amputated. Burn victims may die from infection and the loss of plasma. Skin grafts were developed as a way to prevent such consequences as well as to correct deformities. As early as the sixth century B.C. , Hindu surgeons were involved in nose reconstruction, grafting skin flaps from the patient's nose. Gaspare Tagliacozzo, an Italian physician, brought the technique to Western medicine in the sixteenth century.
Until the late twentieth century, skin grafts were constructed from the patient's own skin (autografts) or cadaver skin (allografts). Infection or, in the case of cadaver skin, rejection were primary concerns. While skin grafted from one part of a patient's body to another is immune to rejection, skin grafts from a donor to a recipient are rejected more aggressively than any other tissue graft or transplant. Although cadaver skin can provide protection from infection and loss of fluids during a burn victim's initial healing period, a subsequent graft of the patient's own skin is often required. The physician is restricted to what skin the patient has available, a decided disadvantage in the case of severe burn victims.
In the mid-1980s, medical researchers and chemical engineers, working in the fields of cell biology and plastics manufacturing, joined forces to develop tissue engineering to reduce the incidences of infection and rejection. One of the catalysts for tissue engineering was the growing shortage of organs available for transplantation. In 1984, a Harvard Medical School surgeon, Joseph Vacanti, shared his frustration over the lack of available livers with his colleague Robert Langer, a chemical engineer at the Massachusetts Institute of Technology. Together, they pondered whether new organs could be grown in the laboratory. The first step was to duplicate the body's production of tissue. Langer came up with the idea of constructing a biodegradable scaffolding on which skin cells could be grown using fibroblasts, cells extracted from donated neonatal foreskins removed during circumcision.
In a variation of this technique developed by other researchers, the extracted fibroblasts are added to collagen, a fibrous protein found in connective tissue. When the compound is heated, the collagen gels and traps the fibroblasts, which in turn arrange themselves around the collagen, becoming compact, dense, and fibrous. After several weeks, keratinocytes, also extracted from the donated foreskins, are seeded onto the new dermal tissue, where they create an epidermal layer.
An artificial skin graft offers several advantages over those derived from the patient and cadavers. It eliminates the need for tissue
Raw Materials
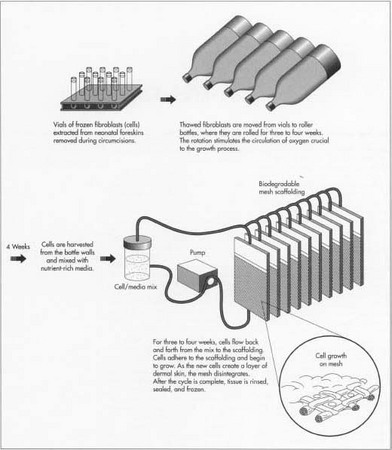
The raw materials needed for the production of artificial skin fall into two categories, the biological components and the necessary laboratory equipment. Most of the donated skin tissue comes from neonatal foreskins removed during circumcision. One foreskin can yield enough cells to make four acres of grafting material. Fibroblasts are separated from the dermal layer of the donated tissue. The fibroblasts are quarantined while they are tested for viruses and other infectious pathogens such as IIV, hepatitis B and C, and mycoplasma. The mother's medical history is recorded. The fibroblasts are stored in glass vials and frozen in liquid nitrogen at -94°F (-70°C). Vials are kept frozen until the fibroblasts are needed to grow cultures. In the collagen method, keratinocytes are also extracted from the foreskin, tested, and frozen.
If the fibroblasts are to be grown on mesh scaffolding, a polymer is created by combining molecules of lactic acid and glycolic acid, the same elements used to make dissolving sutures. The compound undergoes a chemical reaction resulting in a larger molecule that consists of repeating structural units.
In the collagen method, a small amount of bovine collagen is extracted from the extensor tendon of young calves. The collagen is mixed with an acidic nutrient, and stored in a refrigerator at 39.2°F (4°C).
Laboratory equipment includes glass vials, tubing, roller bottles, grafting cartridges, molds, and freezers.
The Manufacturing
Process
The manufacturing process is deceptively simple. Its main function is to trick the extracted fibroblasts into believing that they are in the human body so that they communicate with each other in the natural way to create new skin.
Mesh scaffolding method
- 1 Fibroblasts are thawed and expanded. The fibroblasts are transferred from the vials into roller bottles, which resemble liter soda bottles. The bottles are rotated on their sides for three to four weeks. The rolling action allows the circulation of oxygen, essential to the growth process.
- 2 Cells are transferred to a culture system. The cells are removed from the roller bottles, combined with a nutrient-rich media, flowed through tubes into thin, cassette-like bioreactors housing the biodegradable mesh scaffolding, and sterilized with e-beam radiation. As the cells flow into the cassettes, they adhere to the mesh and begin to grow. The cells are flowed back and forth for three to four weeks. Each day, leftover cell suspension is removed and fresh nutrient is added. Oxygen, pH, nutrient flow, and temperature are controlled by the culture system. As the new cells create a layer of dermal skin, the polymer disintegrates.
- 3 Growth cycle completed. When cell growth on the mesh is completed, the tissue is rinsed with more nutrient-rich media. A cryoprotectant is added. Cassettes are stored individually, labeled, and frozen.
Collagen method
- 4 Cells are transferred to a culture system. A small amount of the cold collagen and nutrient media, approximately 12% of the combined solution, is added to the fibroblasts. The mixture is dispensed into molds and allowed to come to room temperature. As the collagen warms, it gels, trapping the fibroblasts and generating the growth of new skin cells.
- 5 Keratinocytes added. Two weeks after the collagen is added to the fibroblasts, the extracted keratinocytes are thawed and seeded onto the new dermal skin. They are allowed to grow for several days and then exposed to air, inducing the keratinocytes to form epidermal layers.
- 6 Growth cycle completed. The new skin is stored in sterile containers until needed.
The Future
The medical profession is using artificial skin technology to pioneer organ reconstruction. It is hoped that this so-called engineered structural tissue will, for example, someday replace plastic and metal prostheses currently used to replace damaged joints and bones. Ears and noses will be reconstructed by seeding cartilage cells on polymer mesh. The regeneration of breast and urethral tissues is currently under study in the laboratory. Through this technology, it is possible that one day, livers, kidneys, and even hearts, will be grown from human tissues.
Where to Learn More
Periodicals
Langer, Robert and Joseph P. Vacanti. "Artificial Organs," Scientific American, September 1995, pp. 130-133.
Langer, Robert and Joseph P. Vacanti. "Tissue Engineering," Science, May 14, 1993, pp. 920-921.
McCarthy, Michael. "Bio-engineered tissues move towards the clinic," The Lancet, August 17, 1996, p. 466.
Rundle, Rhonda L. "Cells 'Tricked' To Make Skin For Burn Cases," The Wall Street Journal, March 17, 1994.
— Mary F. McNulty
Comment about this article, ask questions, or add new information about this topic: