Hair Dye
Background
Hair dye is one of the oldest known beauty preparations, and was used by ancient cultures in many parts of the world. Records of ancient Egyptians, Greeks, Hebrews, Persians, Chinese, and early Hindu peoples all mention the use of hair colorings. Early hair dyes were made from plants, metallic compounds, or a mixture of the two. Rock alum, quicklime, and wood ash were used for bleaching hair in Roman times, and herbal preparations included mullein, birch bark, saffron, myrrh, and turmeric. Henna was known in many parts of the world; it produces a reddish dye.
Many different plant extracts were used for hair dye in Europe and Asia before the advent of modern dyes. Indigo, known primarily as a fabric dye, could be combined with henna to make light brown to black shades of hair dye. An extract of the flowers of the chamomile plant was long used to lighten hair, and this is still used in many modern hair preparations. The bark, leaves, or nutshells of many trees were used for hair dyes. Wood from the brazilwood tree yielded brown hair dyes, and another hair dye known in antiquity as fustic was derived from a tree similar to the mulberry. Other dyes were produced from walnut leaves or nut husks, and from the galls, a species of oak trees. Some of these plant-derived dyes were mixed with metals such as copper and iron, to produce more lasting or richer shades.
The golden red hair captured by many Renaissance painters was artificially produced by some women. The Italian recipe was to comb a solution of rock alum, black sulfur, and honey through the hair and then let the hair dry in sunlight. Other hair dyes, dating from the sixteenth century, were preparations of lead, quicklime, and salt, or silver nitrate in rose water. Another early method of coloring hair was to apply powder. Pure white powder for hair or wigs was the mark of aristocratic dress in Europe during the seventeenth and eighteenth centuries. White powder was made of wheat starch or potato starch, sometimes mixed with plaster of paris, flour, chalk, or burnt alabaster. Similarly colored powders were sometimes used as well. These were made by adding natural pigments such as burnt sienna or umber to white powder to make brown, and India ink was sometimes used to make black powder. In Biblical times, people used powdered gold on their hair. The use of powdered gold and silver returned briefly as a fad in Europe among the wealthy in the mid-nineteenth century. Other hair colorants were blocks similar to crayons made with wax, soap, and pigments. These could be wetted and rubbed on the hair, or applied with a wet brush.
Preparations such as these were the only hair dyes available until the late nineteenth century. Hydrogen peroxide was discovered in 1818, but it was not until 1867 that it was exhibited at the Paris Exposition as an effective hair lightener. A London chemist and a Parisian hairdresser began marketing a 3% hydrogen peroxide formula at the Exposition as eau de fontaine de jouvence golden (golden fountain of youth water), and this was the first modern chemical hair colorant. Advances in chemistry led to the production of more hair dyes in the late nineteenth century. The first synthetic organic hair dye developed was pyrogallol, a
A French hairdresser, Gaston Boudou, first marketed a standardized range of hair dyes in 1910. Whereas earlier hair colors had been mixed on the spot by hair dressers, and the colors produced were variable, Boudou's dyes produced a predictable color. Sold in a range of 18 colors, from black to light blond, these became very popular both in Europe and in the United States. The amino dyes, however, caused allergic reactions in a significant portion of users. Researchers in the United States are credited with creating a modified, less toxic amino-based hair dye, for standardizing the method of applying the dye, and for establishing strict specifications for the purity and strength of the raw materials. Further advances in hair dye chemistry were made by the makers of Clairol. Clairol produced the first one-step hair dye in 1950. This eliminated the time-consuming preliminary shampoo and pre-lightening that was the established hair-dying protocol. With intensive marketing of this easy-to-use product, the percentage of women in the United States who dyed their hair grew from approximately 8% to almost 50% by 1973.
Raw Materials
Most commercial hair dye formulas are complex, with dozens of ingredients, and the formulas differ considerably from manufacturer to manufacturer. In general, hair dyes include dyes, modifiers, antioxidents, alkalizers, soaps, ammonia, wetting agents, fragrance, and a variety of other chemicals used in small amounts that impart special qualities to hair (such as softening the texture) or give a desired action to the dye (such as making it more or less permanent). The dye chemicals are usually amino compounds, and show up on hair dye ingredient lists with such names as 4-amino-2-hydroxytoluene and m-Aminophenol. Metal oxides, such as titanium dioxide and iron oxide, are often used as pigments as well.
Other chemicals used in hair dyes act as modifiers, which stabilize the dye pigments or otherwise act to modify the shade. The modifiers may bring out color tones, such as green or purple, which complement the dye pigment. One commonly used modifier is resorcinol, though there are many others. Antioxidants protect the dye from oxidizing with air. Most commonly used is sodium sulfite. Alkalizers are added to change the pH of the dye formula, because the dyes work best in a highly alkaline composition. Ammonium hydroxide is a common alkalizer. Beyond these basic chemicals, many different chemicals are used to impart special qualities to a manufacturer's formula. They may be shampoos, fragrances, chemicals that make the formula creamy, foamy, or thick, or contribute to the overall action of the formula.
Hair dyes are usually packaged with a developer, which is in a separate bottle. The developer is most often based on hydrogen peroxide, with the addition of small amounts of other chemicals depending on the manufacturer.
The Manufacturing
Process
Checking ingredients
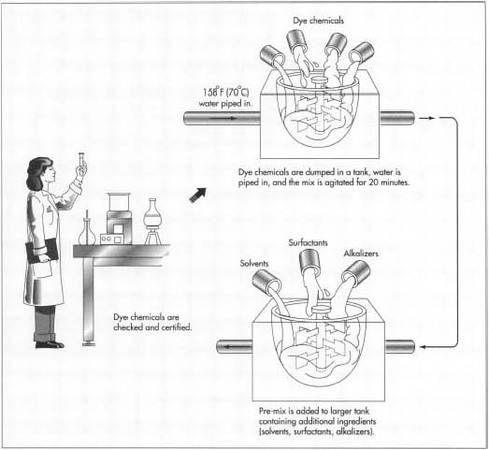
- 1 Before a batch of hair dye is made, the ingredients must be certified. That is, the chemicals must be tested to make sure they are what they are labeled, and that they are the proper potency. Certification may be done by the manufacturer in-house. In many cases, the ingredients arrive from a reputable distributor who has provided a Certificate of Analysis, and this satisfies the manufacturer's requirements.
Weighing
- 2 Next a worker weighs out the ingredients for the batch. For some ingredients, only a small amount is necessary in the batch. But if a very large batch is being made, and several ingredients are needed in large amounts, these may be piped in from storage tanks.
Pre-mixing
- 3 In some hair dye formulas, the dye chemicals are pre-mixed in hot water. The dye chemicals are dumped in a tank, and water which has been already heated to 158°F(70°C) is pumped in. Other ingredients or solvents may also be added to the pre-mix. The pre-mix is agitated for approximately 20 minutes.
Mixing
-
4 The pre-mix is then added to a larger tank, containing the other ingredients of the hair dye. In a small batch, the tanks used may hold about 1,600 lbs (725 kg), and they are portable. A worker wheels the pre-mix tank to the second mix tank and pours the ingredients in. For a very large batch, the tanks may hold 10 times as much as the portable tanks, and in this case they are connected by pipes.
In a formula in which no pre-mixing is required, after checking and weighing, the in gredients go directly to the mixing step. The ingredients are simply mixed in the tank until the proper consistency is reached.
If a heated pre-mix is used, the second mix solution must be allowed to cool. The ingredients that follow the pre-mix may be additional solvents, surfactants, and alkalizers. If the formula includes alcohol, it is no added until the mix reaches 104°F(40°C), so that it does not evaporate. Fragrances too are often added at the end of the mix.
Filling
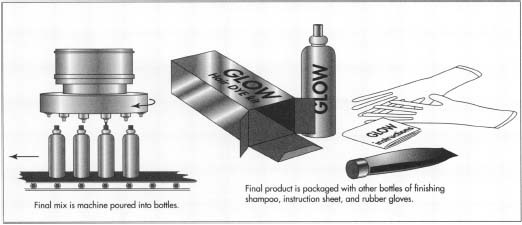
- 5 The finished batch of hair dye is then piped or delivered to a tank in the filling area. A nozzle from this tank lets a measured amount of hair dye into bottles, moving beneath it on a belt. The filled bottles continue on the belt to machines, which affix labels and cap them.
Packaging
- 6 From the filling area, the bottles are taken to the packaging line. At the packaging line, the hair dye bottle is put in a box, together with any other elements such as a bottle of developer or special finishing shampoo, instruction sheet, and gloves and cap, or any other tools provided for the consumer. After the package is complete, it is put in a shipping carton. The full cartons are then taken to the warehouse to await distribution.
Quality Control
Government regulations control what ingredients may be used in hair dyes, as many of them are toxic. Industry researchers will have already tested a formula numerous times in the laboratory before it reaches the manufacturing stage, to make sure a formula is non-irritating, works well, performs consistently, etc. As part of the manufacturing process, workers check their chemicals before they go into a batch, to make sure only the correct chemicals at the correct potency are used. After the batch is mixed, samples are taken, and these are subjected to a series of standard tests. Lab technicians make sure that the batch is the required viscosity and pH balance, and they will also test the dye's action on a swatch of hair. If a hair dye formula is being made for the first time, or if a formula has been altered, technicians will also test samples of the dye after the filling stage.
The Future
Hair dye manufacturers are increasing their use of computers to control and automate the manufacturing process. Computers can be used to weigh and measure ingredients, to control reactions, and to regulate equipment such as pumps. The future may see more fully automated manufacturers and increased efficiency.
Where to Learn More
Books
Balsam, M.S. and Edward Sagarin. Cosmetics Science and Technology. John Wiley & Sons, 1972.
Periodicals
Foltz-Gray, Dorothy. "Declare Your Right to Dye." Health, May-June 1996, pp. 54-57.
"Hair Dye Study." FDA Consumer, May 1994, p. 4.
— Angela Woodward
Comment about this article, ask questions, or add new information about this topic: