Birth Control Pill
Background
Oral contraceptives, or birth control pills, have been used by more than 60 million women worldwide, and are considered by many to be the most socially significant medical advance of the twentieth century. The birth control pill is a tablet taken daily by a woman to prevent pregnancy. The birth control pill does this by inhibiting the development of the egg in the woman's ovary during her monthly menstrual cycle. During a woman's menstrual cycle, a low estrogen level normally triggers the pituitary gland to send out a hormone that initiates development of an egg. The birth control pill releases enough synthetic estrogen to keep that hormone from being released during the monthly cycle. The birth control pill also contains a second synthetic hormone, progestin, which increases the thickness of cervical mucus and impedes development of the uterine lining to further prevent pregnancy. Studies have shown that the birth control pill is 99% effective in preventing pregnancy. The results of studies on the safety of the birth control vary. Some studies show that its use increases the risk of certain types of cancer, while others show that risk to be minimal. There are also claims that the birth control pill increases risk of stroke and heart attacks.
History
The Planned Parenthood Federation of America commissioned Dr. Gregory Pincus and Dr. John Rock to develop a simple and reliable form of contraception in 1950. Over the next several years, the doctors worked on formulating a birth control pill at the Worcester Foundation for Experimental Biology in Massachusetts. They tested their invention on 6,000 women in Puerto Rico and Haiti. The invention was then marketed in the United States in 1960 as Enovid-10.
Many attribute the changing social land-scape in the United States during the 1960s to the widespread acceptance and use of the birth control pill. As sexual relations outside of marriage and for reasons other than childbearing became more socially acceptable and women seeking careers sought family planning methods, the environment was ripe for introduction of this discreet, easy-to-use form of contraception.
Despite its popularity, soon after the birth control pill was introduced, the public began to raise concerns about side effects and safety. As early as 1961, reports had begun to circulate that the birth control pill increased a woman's risk of suffering a stroke or a heart attack by causing blood clotting. In 1965, the federal Food and Drug Administration (FDA) provided a scientist at Johns Hopkins School of Hygiene and Public Health to study the side effects of the birth control pill. The agency also established an Advisory Committee on Obstetrics and Gynecology to study the relationship between oral contraceptives and blood clotting, as well as whether the birth control pill increased risk of breast, cervical, or endometrial cancer. The committee, the first-ever advisory committee established by the FDA, reported in 1966 that it had found no evidence to render the birth control pill unsafe for human use.
Unsatisfied, the FDA called for a larger study of the effects of the birth control pill on blood clotting. The agency also determined,
An oral contraceptive containing only progestin was introduced in the early 1970s. Dubbed the mini-pill, this form of oral contraceptive prevented pregnancy solely by causing changes in the uterus and cervix. An egg was produced, but the changes caused by the mini-pill made it difficult for the egg to unite with sperm from the male. While the mini-pill eliminates the risks posed by estrogen, it has been found to be less effective in preventing pregnancy than pills containing estrogen. Throughout the 1970s, pills containing consistently lower doses of estrogen were introduced on the market.
In 1982 a biphasic birth control pill was introduced, followed by a triphasic pill in 1984. These low-dose pills contained varying ratios of progestin to estrogen. In 1988 all three drug companies still manufacturing high-dose birth control pills withdrew their high-dose products from the market, at the FDA's request. By 1990, the amount of estrogen in birth control pills had been reduced by at least two-thirds. Studies show that the risk of blood clotting in women taking the birth control pill has decreased accordingly. Further studies have shown that high-dose birth control pills actually reduced a woman's risk of ovarian and endometrial cancers, benign cysts of the ovaries and breasts, and pelvic inflammatory disease. The risk of breast or cervical cancer is still disputed.
The pill is still unsafe for certain groups of women, including those who smoke; are obese; have a history of health problems
In addition to preventing pregnancy, the birth control pill can also relieve symptoms associated with premenstrual syndrome. There are at least 30 varieties of birth control pills on the market today.
Rvaw Materials
The main ingredients of the birth control pill are powders containing synthetic versions of the hormones estrogen and progestin.
The Manufacturing
Process
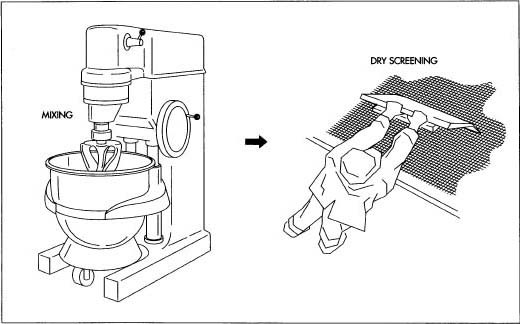
- Using a process known as the wet granulation method, the active ingredients—the powders containing synthetic versions of estrogen and progestin—are mixed together with a dilutant and a disintegrant (products that dilute the powders and cause them to dissolve in liquid) in a large mixer resembling the mixmaster found in many kitchens. For larger batches, a device known as a twin-shell blender may be used.
- Solutions carrying a binding agent (the material that will cause the contents of the tablet to cohere) are stirred into the powder mass, which is wetted until it takes on the consistency of brown sugar.
- The powder mass (known as wet granulation) is forced through a mesh screen.
- The moist material is then placed on shallow trays covered with large sheets of paper and placed in drying cabinets.
- A lubricant, in the form of a fine powder, is screened onto the dried material (known as dry granulation).
- The lubricant and the dry granulation are then mixed in a blender, using a turnbling-type action.
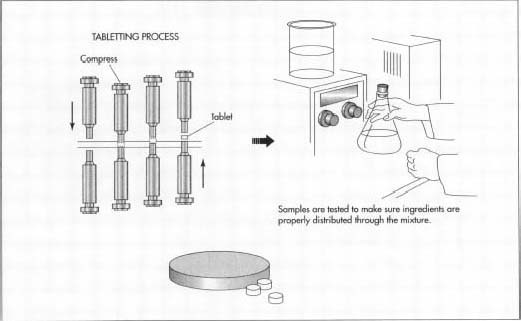
- Tablets are formed from the mixture, typically using a method known as direct compression. Direct compression uses steel punches and dies in large machines, which press tablets directly from the powdered mixture. The physical composition of the powdered mixture is not altered in any way. The punch and die system is often computerized.
- The tablets are inspected to ensure compliance with federal regulations and packaged for shipment to pharmacies.
Quality Control
Like medications, birth control pills are subject to strict regulations set forth by the FDA. Production of birth control pills occurs in a highly sterile environment and samples are taken throughout the production process to ensure each batch of pills meets federal regulations. Factors examined include weight, coloration, and other cosmetic concerns. Many computerized tablet machines can provide weight information. The tablet punch and die systems are regularly inspected as well. In addition, the environment in which the tablets are produced is heavily controlled to avoid the influx of contaminants.
The Future
A relatively recent innovation in the field of birth control is the introduction of Norplant, a contraceptive that works on the same time-release concept as the pill but is inserted under the skin of the upper arm and releases the proper dosage into the body's system each day. Another innovation that is new in the United States, although it has been used in Europe, is RU486, a type of birth control that can be taken after intercourse to prevent pregnancy.
Where to Learn More
Books
Gennaro, Alfonso R., ed. Remington: The Science and Practice of Pharmacy. Eaton, PA: Mack Publishing Co., 1995.
Harris, Carla D. "The Birth Control Pill Revisited." In NAACOG's Clinical Issues, 1992: pp. 246-50.
Ketzung, Bertram. Basic and Clinical Pharmacology. Stamford, CT: Appleton and Lange, 1998.
— Kristin Palm