Antiperspirant/
Deodorant Stick
Background
Antiperspirant/deodorant (APD) sticks are used to reduce underarm wetness and control body odor. These products are made by blending active ingredients with waxes, oils, and silicones and molding the mixture into stick form.
Body odor is primarily generated in the area under the arms where there is a high concentration of sweat glands. While sweat from these glands is initially odorless, it contains natural oils, called lipids, that provide a growth medium for bacteria living on the skin. These bacteria interact with the lipids, converting them into compounds that have a characteristic sweaty odor. Isovaleric acid, for example, is one chemical compound that gives sweat its smell.
There are two primary types of products used to control body odor. The first, deodorants, reduce body odor by killing the odor-causing bacteria. These products do not affect the amount of perspiration the body produces. Antiperspirants, on the other hand, inhibit the activity of sweat glands so less moisture is produced. In addition to avoiding unpleasant wetness, these products also decrease odor because there is less sweat for the bacteria to act upon. While deodorants are considered to be cosmetic products because they only control odor, antiperspirants are actually drugs because they affect the physiology of the body. Although the exact mechanism of this physiological interaction is not fully understood, theory has it that antiperspirant salts form temporary plugs in some of the sweat gland openings so that moisture is not secreted. While this moisture reduction is not severe enough to interfere with normal body metabolism, it does noticeably lessen underarm wetness.
History
Products to control body odor and wetness have been used for centuries. Before bathing became commonplace, people used heavy colognes to mask body odor. In the late nineteenth century, chemists developed products that were able to prevent the formation of these odors. Early antiperspirants were pastes that were applied to the underarm area; the first such product to be trademarked in the United States was Mum in 1888. It was a waxy cream that was difficult to apply and extremely messy. A few years later, Everdry, the first antiperspirant to use aluminum chloride was developed. Within 15 years, a variety of products were marketed in a number of different forms including creams, solids, pads, dabbers, roll-ons, and powders.
In the late 1950s, manufacturers began using aerosol technology to dispense personal care products such as perfumes and shaving creams. In the early 1960s, Gillette introduced Right Guard, the first aerosol antiperspirant. Aerosols became a popular way to dispense antiperspirants because they allowed the user to apply without having to touch the underarm area. By 1967, half the antiperspirants sold in the United States were in aerosol form, and by the early 1970s, they accounted for 82% of all sales.
However, later that decade two technical issues arose which greatly impacted the popularity of these products. First, in 1977, the Food and Drug Administration (FDA) banned the primary active ingredient used in aerosols, aluminum zirconium complexes,
As the popularity of aerosols waned, antiperspirants in stick form became increasingly popular. In 1974, sticks held only about 4% of the market and they were considered to be wet and aesthetically unpleasing. Such products were generally associated with deodorants for men. Because of breakthroughs in ingredient technology that allowed for drier, more efficacious products, sticks gained acceptance between 1974-1978. Consumers embraced sticks as an alternative to aerosols and their market share swelled to over 35% by the mid 1980s. Today, sticks are the single most popular antiperspirant form.
Raw Materials
Antiperspirants consist of the active drug ingredients that control perspiration; gelling agents that form the stick matrix; and other ingredients, such as fragrance or colorants, that make the product aesthetically pleasing.
Active ingredients
The Food and Drug Administration (FDA) controls the active ingredients used in antiperspirants because they are legally classified as drugs. The FDA publishes an Over the Counter (OTC) Drug monograph that lists which ingredients are approved for use. The ingredients on this list are limited to aluminum chlorohydrate, aluminum chloride, aluminum sulfate, and aluminum zirconium complexes. Of these compounds, the most commonly used is aluminum zirconium tetrachlorohydrex glycine. Most of these materials are supplied as powders, and they are typically used at levels of 8-25% based on the weight of the finished product.
Gelling agents
The bulk of the formulation consists of waxy or fatty materials that are gelled to form a solid stick. Common examples include stearyl alcohol, cetyl alcohol, hydrogenated castor oil, and glyceryl stearate. These waxy materials are blended with lubricating oils and emollients such as cyclomethicone, which is a volatile silicone compound. These silicones are liquids at room temperature, but they quickly evaporate and are used because they leave the skin feeling smooth and dry. In addition, talc, starches, or other powders may be added to control stick consistency and to give the product a dry feel and a smooth payoff.
Other ingredients
Fragrance and colorants may be added to the formula to improve its odor or appearance. Some brands have fragrances that are time released. Other brands may add featured ingredients that contribute little functionality but are designed to increase consumer appeal.
The Manufacturing
Process
Batching
- 1 In the batching process, ingredients are combined in a jacketed stainless steel kettle. Steam heat is applied to melt the ingredients while the batch is being mixed. During the blending process, the temperature must be carefully controlled to avoid scorching the waxy ingredients. Once all the ingredients have been added to the batch, it is blended until uniform.
Filling
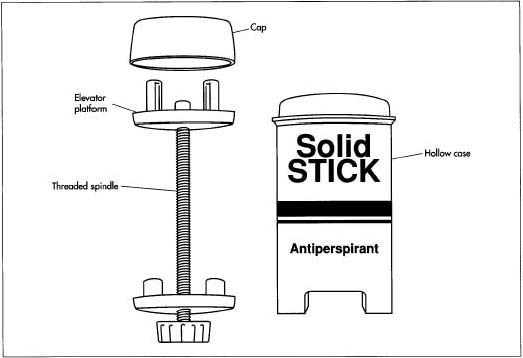
- 2 Stick packages are typically hollow tubes with an elevator platform inside that moves up and down to dispense the product. In some packages, this platform can be pushed up by hand, in others it is elevated by turning a screw that causes it to travel up along a central threaded post. These empty containers move along a conveyor belt where the molten product is dispensed through a filling nozzle. The exact process varies depending on whether the package is designed to be filled from the top or bottom. In general, the product is filled slightly above its congealing temperature so that it flows easily. If it is filled too hot, the dispersed solids may settle to the bottom; if it is filled too cold, air bubbles will be trapped in the stick.
Finishing operations
- 3 Sticks may then go through subsequent finishing operations to ensure the surface is smooth and that they are free from trapped pockets of air. These operations usually involve heating the tops of the sticks slightly by passing them under an infrared lamp.
- 4 A probe is then stuck into the center of the stick to allow air to escape and the surface is heated again to remelt the product, allowing it to flow into the void.
- 5 At the next station, the sticks pass through a refrigeration tunnel that rapidly lowers the temperature and forces them to solidify. Depending on the package design, a top or bottom piece is put into place to seal the container.
- 6 Finally, the sticks may pass through cleaning stations before they are placed in cartons for shipping.
Quality Control
Safety testing
Safety testing guidelines are recommended by the Cosmetics, Toiletries, and Fragrance Association (CTFA), the primary trade organization for the cosmetic industry. While these guidelines are not absolute rules, they do give manufacturers an indication of the minimal level of testing that should be done to ensure their products are safe. These tests include evaluation of the irritation potential (for skin and eyes), contact sensitization (where contact with the product can result in a chemical delayed reaction), photodermatitis (where light interacts with the product to cause a reaction), as well as toxicity (both ingested and inhaled.)
Efficacy testing
According to the OTC monograph, antiperspirants must reduce the amount of perspiration by at least 20% and a variety of test methods are used to ensure formulations meet this requirement. One method, known as the visualization technique, shows the action of the sweat glands via a color change. This is done by first painting the skin with a mixture of iodine castor oil and alcohol. After drying, the skin is then whitened with a layer of powdered starch. When sweat droplets are exuded, they appear as very dark spots against the white background. Another method involves painting a silicone polymer painted onto the skin to form a film. The subject is made to sweat by exposure to elevated temperature or by physical exertion and the film is peeled off and examined for tiny holes formed by the sweat drops. A relative measure of the amount of sweat produced by the body can be obtained by counting the number of holes in the film. Sweat production can also be measured using infrared gas sensors that detect moisture loss. In this process, a constant stream of gas is passed over the subject's armpits and is subsequently analyzed for moisture content. Gravimetric techniques are also used to measure the amount of sweat collected on cotton balls.
Byproducts/Waste
During the filling process, overfilling or spillage may occur, resulting in scrap product. This can usually be returned to the batch tank and remelted. Depending on the quantity of material involved and the degree of reheating, the batch may have to be assayed to ensure it still meets quality specifications. Additional solvent or fragrance may be added to replace that which was driven off during the reheating operation. The product can then be filled into the packages. Any waste material that is contaminated or otherwise unsuited for refilling must be disposed of in accordance with local regulations.
The Future
Clear APD sticks have gained popularity in the 1990s. Although the first clear stick products appeared as early as 1979, these early products had stability problems and did not have a significant impact on the market. Since the late 1970s, chemists for various companies have struggled to advance clear APD stick technology. It wasn't until 1993, when Bristol Myers introduced Ban for Man, that clear stick products achieved significant commercial success. It is also interesting to note earlier that year Gillette began spending millions of dollars in advertising for the launch of its Cool Wave clear APD gel-stick. This product was actually a gel, but it was dispensed from a stick-type package. The long term market impact of clear APD sticks and gel-sticks remains to be seen.
Where to Learn More
Books
Laden, Karl. Antiperspirants and Deodorants New York: Marcel Dekker, 1988.
Periodicals
"Flexibility is the hallmark of fluid packag ing." Drug & Cosmetic Industry (June 1996): 98.
"Gillette deodorants are fit to fill upside-down." Packaging Digest (September 1993): 54.
Kintish, Lisa. "A Clear Advantage. The emergence of clear technology has given a much-needed boost to the antiperspirant and deodorant market." Soap-Cosmetics-Chemical Specialties (July 1997): 29.
Springer, Neil and Helga Tilton. "Staying Power. "Chemical Marketing Reporter (August 9, 1993): SR12.
Strandberg, Keith. "Antiperspirant & Deodorant Update." Soap-Cosmetics-Chemical Specialties (April 1993): 30.
— Randy Schueller
how can i make alum deodorant stick ? ammonium alum and potassium alum is use in this product , it was already selling in market but i want to make by myself.
yours sincerly
joseph carter