Bioceramics
Background
Over the last several decades, bioceramics have helped improve the quality of life for millions of people. These specially designed materials—polycrystalline aluminum oxide, hydroxyapatite (a mineral of calcium phosphate that is also the major component of vertebrate bone), partially stabilized zirconium oxide, bioactive glass or glass-ceramics, and polyethylene-hydroxyapatite composites—have been successfully used for the repair, reconstruction, and replacement of diseased or damaged parts of the body, especially bone. For instance, aluminum oxide has been used in orthopedic surgery for more than 20 years as the joint surface in total hip prostheses because of its exceptionally low coefficient of friction and minimal wear rates.
Clinical success requires the simultaneous achievement of a stable interface with connective tissue and a match of the mechanical behavior of the implant with the tissue to be replaced. Bioceramics, made from a calcium phosphate material containing tiny pores, have been used to coat metal joint implants or used as unloaded space fillers for bone ingrowth. Ingrowth of tissue into the pores occurs, with an increase in interfacial area between the implant and the tissues and a resulting increase in resistance to movement of the device in the tissue. As in natural bone, proteins adsorb to the calcium phosphate surface to provide the critical intervening layer through which the bone cells interact with the implanted biomaterial.
Resorbable biomaterials have also been designed to degrade gradually over time to be replaced by the natural host tissue. Porous or particulate calcium phosphate ceramic materials (such as tricalcium phosphate) have been successfully used as resorbable materials for low mechanical strength applications, such as repairs of the jaw or head. Resorbable bioactive glasses are also replaced rapidly with regenerated bone.
Bioactive materials form a biologically active layer on the surface of the implant, which result in the formation of a bond between the natural tissues and the material. A wide range of bonding rates and thickness of interfacial bonding layers are possible by changing the composition of the bioactive material.
Bioactive materials include glass and glass-ceramics based on silicon dioxide-phosphate systems containing apatite (a natural calcium phosphate containing some fluorine or chlorine), dense synthetic hydroxyapatite, and polyethylene-hydroxyapatite composites. Applications include orthopedic implants (vertebral prostheses, intervertebral spacers, bone grafting), middle-ear bone replacements, and jawbone repair. Bioactive glass and glass-ceramic implants have been used for more than 10 years in the middle-ear application. Bioactive glass particles have also been used as fillers around teeth that have had gum disease, preventing the teeth from falling out.
Design
The performance of the artificial bone depends on its composition and end use application. Careful selection of the right material with suitable properties is thus important. Computer-aided design software is also used for optimizing the shape and for simulating the mechanical behavior of the implant with the surrounding bone tissue. A mathematical technique called finite element analysis is used to determine the stress distribution on both the implant and biological structure. Prototypes are then fabricated that undergo testing of properties, as well as clinical tests, before final production.
Raw Materials
The major raw material is usually a ceramic powder of specific composition and high purity. Additives include binders, lubricants, and other chemicals to assist in the shape forming process. The powder may also contain a sintering aid, which helps the ceramic material to densify properly during firing and sometimes at a lower temperature. If a chemical-based process is used, organic precursors and solvents are combined into a solution to make the final product.
The Manufacturing
Process
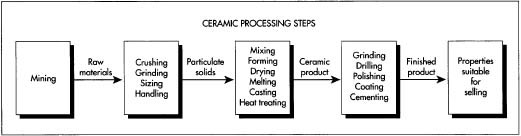
Depending on its composition, artificial bone is made using two processes, the traditional ceramic process and a chemical-based method called sol gel. In the sol gel method, two approaches can be used. In one, a suspension of extremely small particles is allowed to gel inside a mold, followed by aging at 77-176° F (25-80° C) for several hours, drying, and several thermal treatments to chemically stabilize and densify the material. The other approach uses a solution of chemical precursors as the starting material followed by the same process. Since the ceramic process is more common, it will be discussed in more detail here.
Raw material preparation
- 1 The ceramic powder is manufactured elsewhere from mined or processed raw materials. Additional crushing and grinding steps may be necessary to achieve the desired particle size. The ceramic powder plus additives are carefully weighed in the appropriate amounts and then mixed in some type of mixing machine equipped with blades or revolving rolls. Sometimes mixing and particle size reduction takes place at the same time, using a milling machine. A ball mill uses rotating cylinders filled with the mixture and spherical media to disperse the material and reduce its particle size. An attrition mill uses tiny beads and rotating agitators to accomplish the same thing.
Forming
- 2 After mixing, the ceramic material is of plastic consistency and now ready for forming into the desired shape. A variety of methods can be used, including injection molding, extrusion, or pressing. In injection molding, the mix is loaded into a heated cylinder, where it softens. A steel piston forces the hot mixture into a cooled metal mold. Extrusion compacts the material in a high-pressure cylinder and then forces the material out through a specially shaped die orifice. Pressing involves compaction of the material in steel dies or the material is placed in a rubber mold inside a high-pressure oil or water cylinder, with uniform pressure applied. Another variation of pressing called hot pressing combines forming and firing in one step using heated dies.
Drying and firing
- 3 After forming, the ceramic bone must undergo several thermal treatments. The first dries the material to remove moisture using a drying oven or chamber. After drying, a kiln or furnace is used to heat the material at high temperatures in order to remove organics and densify the material. The firing cycle will depend on the material composition and must be designed at the appropriate heating rates to prevent cracking.
Finishing
- 4 After firing, one or more finishing processes may be required depending on application. To achieve the desired dimensional and surface finish specifications, grinding and/or polishing is conducted. Grinding and polishing of the harder materials usually requires diamond tooling or abrasives. Drilling may be needed to form holes of various shapes. If the application requires joining of two or more components, a brazing or cementing method is used.
Quality Control
During the manufacture of the artificial bone material or component, control of each processing step is required to control the properties that affect performance. The
Since artificial bone can sometimes be considered a medical device or at least part of a medical device, it must meet national and international standards for such devices and materials, as well as regulations established
The FDA has the authority to regulate medical devices during most phases of their development, testing, production, distribution and use, with a focus on the pre- and post-market phases to ensure safety and effectiveness. The level of regulation or control is based on how the device is classified (I, II, or III). The higher the class, the more regulation—Class III devices must have an approved Pre-market Approval Application.
All classes are subject to General Controls, which involves registering each manufacturing location, listing marketed medical devices, submitting a Premarket Notification for a new device, and manufacturing the device according to the Good Manufacturing Practices regulation. This regulation includes requirements for the quality assurance program used by the manufacturer.
Byproducts/Waste
Since careful control of the manufacturing process is so important, waste is minimal. Since contamination must be avoided, any waste produced can only be recycled if the properties match the starting material. Sometimes waste material can be used to make other ceramic products of lower quality. Byproducts that must be controlled throughout the process include dust and organic emissions from firing.
The Future
In the next century—as a better understanding of the interactions of artificial bone with organic components is achieved on the molecular level—it will be possible to tailor the physical and chemical properties of the material with the specific biological and metabolic requirements of bone tissues or disease states. Since the population is continuing to grow older, artificial bone will play an even more important role in improving the health of many people around the world.
Where to Learn More
Books
Fischman, Gary and Clare, Alexis, ed. "Bioceramics: Materials and Applications." In Ceramic Transactions. The American Ceramic Society, 1995.
Hench, Larry and June Wilson. An Introduction to Bioceramics. World Scientific Publishing Col. Ltd., 1993.
Ravaglioli, A. and A. Krajewski, ed. Bioceramics and the Human Body. Elsevier Applied Science, 1992.
Periodicals
Hench, Larry. "Bioceramics, 'A Clinical Success."' Ceramic Bulletin. The American Ceramic Society (July 1998): 67-74.
— Laurel Sheppard