Natural Gas
Background
Natural gas is a mixture of combustible gases formed underground by the decomposition of organic materials in plant and animal. It is usually found in areas where oil is present, although there are several large underground reservoirs of natural gas where there is little or no oil. Natural gas is widely used for heating and cooking, as well as for a variety of industrial applications.
History
Natural gas was known to early man in the form of seepages from rocks and springs. Sometimes, lightning or other sources of ignition would cause these gas seepages to burn, giving rise to stories of fire issuing from the ground. In about 900 B.C. natural gas was drawn from wells in China. The gas was burned, and the heat was used to evaporate seawater in order to produce salt. By the first century, the Chinese had developed more advanced techniques for tapping underground reservoirs of natural gas, which allowed them to drill wells as deep as 4,800 ft (1,460 m) in soft soil. They used metal drilling bits inserted through sections of hollowed-out bamboo pipes to reach the gas and bring it to the surface.
The Romans also knew about natural gas, and Julius Caesar was supposed to have witnessed a "burning spring" near Grenoble, France. Religious temples in early Russia were built around places where burning natural gas seepages formed "eternal flames."
In the United States, the first intentional use of natural gas occurred in 1821 when William Hart drilled a well to tap a shallow gas pocket along the bank of Canadaway Creek near Fredonia, New York. He piped the gas through hollowed logs to a nearby building where he burned it for illumination. In 1865, the Fredonia Gas, Light, and Waterworks Company became the first natural gas company in the United States. The first long-distance gas pipeline ran 25 mi (40 km) from a gas field to Rochester, New York, in 1872. It too used hollowed logs for pipes. The development of the Bunsen burner by Robert Bunsen in 1885 led to an interest in using natural gas as a source of heating and cooking, in addition to its use for lighting. In 1891, a high-pressure gas deposit was tapped in central Indiana, and a 120 mi (192 km) pipeline was built to bring the gas to Chicago, Illinois.
Despite these early efforts, the lack of a good distribution system for natural gas limited its use to local areas where the gas was found. Most of the gas that came to the surface as part of oil drilling in more remote areas was simply vented to the atmosphere or burned off in giant flares that illuminated the oil fields day and night. By the 1910s, oil companies realized that this practice was costing them potential profits and they began an aggressive program to install gas pipelines to large metropolitan areas across the United States. It wasn't until after World War II that this pipeline program had reached enough cities and towns to make natural gas an attractive alternative to electricity and coal.
By 2000, there were over 600 natural gas processing plants in the United States connected to more than 300,000 mi (480,000 km) of main transportation pipelines. Worldwide, there are also significant deposits of natural gas in the former Soviet Union, Canada, China, and the Arabian Gulf countries of the Middle East.
Raw Materials
Raw natural gas is composed of several gases. The main component is methane. Other components include ethane, propane, butane, and many other combustible hydrocarbons. Raw natural gas may also contain water vapor, hydrogen sulfide, carbon dioxide, nitrogen, and helium.
During processing, many of these components may be removed. Some—such as ethane, propane, butane, hydrogen sulfide, and helium—may be partially or completely removed to be processed and sold as separate commodities. Other components—such as water vapor, carbon dioxide, and nitrogen—may be removed to improve the quality of the natural gas or to make it easier to move the gas over great distances through pipelines.
The resulting processed natural gas contains mostly methane and ethane, although there is no such thing as a "typical" natural gas. Certain other components may be added to the processed gas to give it special qualities. For example, a chemical known as mercaptan is added to give the gas a distinctive odor that warns people of a leak.
The Manufacturing Process
The methods used to extract, process, transport, store, and distribute natural gas depend on the location and composition of the raw gas and the location and application of the gas by the end users. Here is a typical sequence of operations used to produce natural gas for home heating and cooking use.
Extracting
- 1 Some underground natural gas reservoirs are under enough internal pressure that the gas can flow up the well and reach Earth's surface without additional help. However, most wells require a pump to bring the gas (and oil, if it is present) to the surface. The most common pump has a long rod attached to a piston deep in the well. The rod is alternately pulled upward and plunged back into the well by a beam that slowly rocks up and down on top of a vertical support. This configuration is often called a horse head pump because the shape of the pulling mechanism on the end of the rocking beam resembles a horse's head.
- 2 When the raw natural gas reaches the surface, it is separated from any oil that might be present and is piped to a central gas processing plant nearby. Several hundred wells may all feed into the same plant.
Processing
- 3 About 75% of the raw natural gas in the United States comes from underground reservoirs where little or no oil is present. This gas is easier to process than gas from oil wells. Regardless of the source, most raw natural gas contains dirt, sand, and water vapor, which must be removed before further processing to prevent contamination and corrosion of the equipment and pipelines. The dirt and sand are removed with filters or traps near the well. The water vapor is usually removed by passing the gas through a tower filled with granules of a solid desiccant, such as alumina or silica gel, or through a liquid desiccant, such as a glycol. After it has been cleaned and dried, the raw gas may be processed further or it may be sent directly to a compressor station and pumped into a main transportation pipeline.
- 4 If the raw natural gas contains a large amount of heavier hydrocarbon gases, such as propane and butane, these materials are removed to be sold separately. The most common method is to bubble the raw gas up through a tall, closed tower containing a cold absorption oil, similar to kerosene. As the gas comes in contact with the cold oil, the heavier hydrocarbon gases condense into liquids and are trapped in the oil. The lighter hydrocarbon gases, such as methane and ethane, do not condense into liquid and flow out the top of the tower. About 85% of the propane and almost all of the butane and heavier hydrocarbons are trapped this way. The absorption oil is then distilled to remove the trapped hydrocarbons, which are separated into individual components in a fractionation tower.
-
5 At this point, the natural gas contains methane, ethane, and a small
amount of propane that wasn't trapped. It may also contain
varying amounts of carbon dioxide,
hydrogen sulfide, nitrogen, and other materials. A portion of the ethane is sometimes removed to be used as a raw material in various chemical processes. To accomplish this, the water vapor in the gas is further reduced using one of several methods, and the gas is then subjected to repeated compression and expansion cycles to cool the ethane and capture it as a liquid.
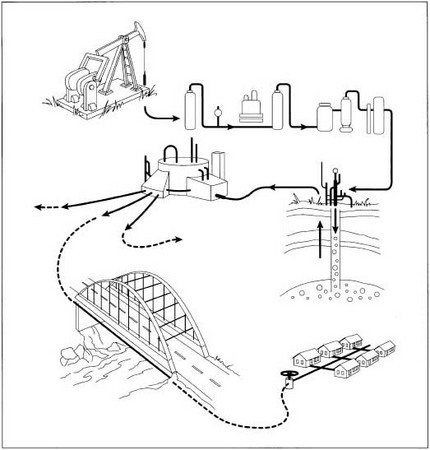 A diagram depicting the production of natural gas from underground source to household usage.
A diagram depicting the production of natural gas from underground source to household usage. - 6 Some natural gas contains a high percentage of carbon dioxide and hydrogen sulfide. These chemicals can react with the remaining water vapor in the gas to form an acid, which can cause corrosion. They are removed by flowing the gas up through a tower while a spray of water mixed with a solvent, such as monoethanolamine, is injected at the top. The solvent reacts with the chemicals, and the solution is drained off the bottom of the tower for further processing.
- 7 Some natural gas also contains a high percentage of nitrogen. Because nitrogen does not burn, it reduces the heating value of the natural gas. After the carbon dioxide and hydrogen sulfide have been removed, the gas goes through a low-temperature distillation process to liquefy and separate the nitrogen. Together, the processes in steps 6 and 7 are sometimes called "upgrading" the gas because the natural gas is now cleaner and will burn hotter.
- 8 If helium gas is to be captured, it is done after the nitrogen is removed. This involves a complex distillation and purification process to isolate the helium from other gases. Natural gas is the primary source of industrial helium in the United States.
Transporting
- 9 Mercaptan is injected into the processed natural gas to give it a distinctive warning odor, and the gas is piped to a compressor station where the pressure is increased to about 200-1,500 psi (1,380-10,350 kPa). The gas is then transported across country through one of several major pipelines installed underground. These pipelines range from 20 to 42 in (51 to 107 cm) in diameter. About every 100 mi (160 km), another compressor boosts the gas pressure to make up for small pressure losses caused by friction between the gas and the pipe walls. This keeps the gas flowing.
- 10 When the pressurized natural gas reaches the vicinity of its final destination, it is sometimes injected back into the ground for storage. Depleted underground gas and oil reservoirs, porous rock layers known as aquifers, or subterranean salt caverns may be used to store the gas. This ensures a ready supply during the colder winter months.
Distributing
- 11 When gas is needed, it is drawn out of underground storage and is transported through pipelines at pressures up to 1,000 psi (6,900 kPa). These pipelines bring the gas into the city or area where it is to be used.
- 12 The pressure is reduced to below 60 psi (410 kPa), and the gas is distributed in underground pipes that run throughout the area. Before the gas is piped into each house or business, the pressure is further reduced to about 0.25 psi (1.7 kPa).
Quality Control
Natural gas burns readily in air and can explode violently if a large quantity is suddenly ignited. Entire buildings have been leveled by powerful blasts resulting from natural gas leaks. In other cases, people have suffocated in closed rooms that slowly filled with natural gas. Because natural gas is odorless, foul-smelling mercaptan is added to the gas so that even a small leak will be immediately noticeable. To protect high-pressure underground gas pipelines, a bright yellow plastic tape is buried in the ground a few feet above the pipeline to warn people who might be digging in the area. That way, they will uncover the tape before they actually strike the pipeline below. Warning signs are also placed at ground level along the entire length of the pipeline as an additional precaution.
The Future
Because natural gas is clean burning, it is being considered as an alternative fuel for motor vehicles. Compressed natural gas (CNG) cars and trucks are already on the road in many areas. Companies using industrial processes that require high temperatures are also turning to natural gas instead of other fuels in order to reduce the air pollution emitted by their plants. This includes companies involved in manufacturing steel, glass, ceramics, cement, paper, chemicals, aluminum, and processed foods.
Where to Learn More
Books
Kroschwitz, Jacqueline I., and Mary Howe-Grant (eds.). "Gas, Natural." In Encyclopedia of Chemical Technology. 4th ed., vol. 12. New York: John Wiley and Sons, Inc., 1993.
Tussing, Arlon R., and Bob Tippee. The Natural Gas Industry: Evolution, Structure, and Economics. 2nd ed. Tulsa, OK: PennWell Publishing, 1995.
Other
Natural Gas Information and Educational Resources. http://www.naturalgas.org (November 1, 2000).
Pacific Gas and Electric Company. "How Our Gas System Works." http://www.pge.com/006_news/006c2gassys.shtml (November 12, 2000).
— Chris Cavette
Once again thanks a lot.