Vermiculite
Background
The term vermiculite applies to a group of minerals characterized by their ability to expand into long, worm-like strands when heated. This expansion process is called exfoliation. The name vermiculite is derived from a combination of the Latin word vermiculare meaning "to breed worms," and the English suffix-ite, meaning mineral or rock. In its expanded form, vermiculite has a very low density and thermal conductivity, which makes it attractive for use as a soil amendment, lightweight construction aggregate, and thermal insulation filler. Expanded vermiculite also has a very large chemically active surface area, which makes it useful as an absorbent in some chemical processes. When vermiculite is ground into a fine powder, it is used as a filler in inks, paints, plastics, and other materials.
History
Vermiculite and its unique properties were known as early as 1824, when Thomas H. Webb experimented with it in Worcester, Massachusetts. It was Webb who gave the mineral its fanciful name because he thought the long strands looked like a mass of small worms. Vermiculite was regarded as not much more than a scientific curiosity until the early 1900s when more practical uses were sought. The first commercial mining effort occurred in 1915 in Colorado. The material was sold as tung ash, but did not find sufficient buyers, and the venture failed. The first successful vermiculite mine was started by the Zonolite Company in Libby, Montana, in 1923. The mine continued to operate until 1990.
The largest vermiculite mining operation in the world is located in the Phalabowra (also sometimes spelled Palabora) district of the Republic of South Africa. Other countries producing significant amounts of vermiculite include the United States, China, Russia, Brazil, Japan, Zimbabwe, and Australia.
In 1999, there were three active vermiculite mining operations in the United States, two in South Carolina and one in Virginia, which shipped concentrated vermiculite ore to exfoliation plants located throughout the country. In addition to using concentrated vermiculite from domestic mining operations, these plants also imported about 77,000 tons (70,000 metric tons) of concentrated vermiculite from foreign sources—mostly South Africa.
Raw Materials
Technically, vermiculite encompasses a large group of hydrated laminar magnesium-aluminum-iron silicates, which resemble mica. There are two keys to the unique properties of vermiculite. The first is its laminar (or layered) crystalline structure, which provides the hinged plates that make the material expand or unfold in a linear manner, like an accordion. The second is the fact that it contains trapped water, which flashes into steam when heated to force the layers open. There are a great many naturally occurring vermiculite minerals and soils, and their identification often requires sophisticated scientific analysis.
One of the most common forms of vermiculite is generally known as commercial vermiculite. This is the form that is mined and processed for various end uses. It is derived from rocks containing large crystals of the minerals biotite and iron-bearing phlogopite. As these rocks are exposed to the weather, they start to decompose, allowing water to enter and react with the various chemicals present. As the decomposition and chemical reactions proceed, vermiculite is formed.
A typical chemical analysis of commercial vermiculite shows it contains 38-46% silicon oxide (SiO 2 ), 16-35% magnesium oxide (MgO), 10-16% aluminum oxide (Al 2 O 3 ), 8-16% water, plus lesser amounts of several other chemicals.
When commercial vermiculite flakes are heated and expanded, they undergo a color change that depends on the chemicals present and the temperature of the furnace. The resulting expanded vermiculite granules are usually a gold-brown color with a bulk density of about 4-10 lb/cu ft (64-160 kg/cu m), depending on the size of the granules.
The Manufacturing Process
The manufacturing process used to produce commercial expanded vermiculite consists of two separate operations. The mining and concentrating operations that produce raw vermiculite flakes are conducted at one location. The exfoliation and classifying operations that produce various sizes of lightweight, expanded vermiculite granules for use in other products are conducted in another location. Sometimes these two locations can be half a world apart.
There are many different methods used in both of these operations. The exact methods vary from mine to mine and plant to plant. Here is a typical manufacturing process used to produce commercial expanded vermiculite.
Mining
- l Rocks containing vermiculite are dug from a huge open pit in the ground. The soil on top of the rocks, called the overburden, is removed with power shovels or earth scrapers. The exposed rock layers are then drilled with large pneumatic or hydraulic drills, and the holes are filled with explosive charges. When all personnel and equipment have been moved out of the area, the explosive charges are detonated.
- 2 The resulting heap of loose rocks are scooped up with power shovels and dumped into trucks or train cars, which carry the rocks to a nearby processing plant.
Concentrating
- 3 The rocks are fed through a series of crushers and screens to reduce their size. The vermiculite is separated from the surrounding rocks and dirt using various wet or dry techniques depending on the particular mining operation and local environmental regulations. These techniques may include froth flotation, gravity separations, winnowing, or electrostatic separation. In each of these techniques, either the vermiculite itself or the other materials are trapped and separated from each other until the resulting vermiculite flakes are about 90% pure by weight.
- 4 The vermiculite flakes extracted from various sections of the mine may be blended together before further processing to ensure uniformity of the product.
Grading
- 5 The separated vermiculite flakes are sorted by size. This may be done with a series of screens or it may be done in a long enclosed wind tunnel . In the wind tunnel, the flakes are fed into the upstream end of the tunnel and are carried along the length of the tunnel by the flow of air. The larger flakes, being heavier, fall out of the air stream first and are caught in a hopper at the bottom of the tunnel. This separation by weight continues down the length of the tunnel until all the flakes are caught in hoppers. By controlling the length of each hopper opening and the velocity of the air, the flakes can be sorted into various sizes, or grades, ranging from about 0.63 in (16 mm) down to about 0.02 in (0.8 mm) in diameter. If the particular vermiculite being mined tends to form a high percentage of large flakes, the flakes may be slightly crushed to delaminate them and reduce their size. This process is called debooking and allows the flakes to be quickly heated during the exfoliation process.
-
6 The graded vermiculite flakes are dumped into large plastic bags or
other containers for shipping to various exfoliation
plants. If the flakes are to be shipped to plants overseas, they are loaded in bulk into the holds of ships for transport.
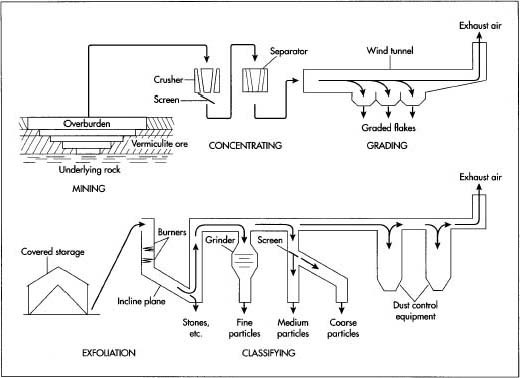 A diagram depicting the processing of vermiculite.
A diagram depicting the processing of vermiculite.
Exfoliating
- 7 The vermiculite flakes are transported by truck or train from the port or mine to the exfoliation plant, where they are offloaded and stored in a covered area to protect them from contaminants and the weather. It is important to prevent the flakes from absorbing moisture. Otherwise, it will take too much energy to heat the flakes to the required temperature to make them expand.
- 8 The flakes are loaded onto a conveyor belt and lifted to the top of a 20-25 ft (6.1-7.6 m) high vertical furnace lined with ceramic bricks. As the flakes fall down the length of the furnace, they pass through one or more burners fired by natural gas . The temperature inside the furnace reaches approximately 1,000-1,500°F (540-810°C), which is sufficient to make the trapped water in the flakes flash to steam and cause the flakes to expand into worm-like particles. At the bottom of the furnace, the particles slide down an inclined plane. This delays the exit of the particles from the furnace and allows the vermiculite to be heated further in order to reach full expansion. Other exfoliation plants may use different furnace configurations, but the general sequence of operations is similar.
Classifying
- 9 The hot, expanded vermiculite particles are then drawn up a vertical tube by a vacuum. Any small stones or other solid contaminants are too heavy to be carried upward by the gentle flow of air and fall out the bottom of the tube. The air flow also acts to cool the hot vermiculite.
- 10 If a customer or application requires fine particles, the vermiculite may be ground and screened to produce a specific size or range of sizes before it is packaged for shipping. In some exfoliation plants, the larger particles may also be screened or sorted into various sizes, depending on the final use.
- 11 The sorted, or classified, vermiculite particles are then deposited into storage hoppers, where they are dispensed into individual 4-6 cu ft (0.10-0.15 cu m) paper or plastic bags for retail sales or placed into larger 50 cu ft (1.3 cu m) bags for use in various commercial applications. The bags are sealed, labeled, and moved to a warehouse for shipping.
Health Aspects
Vermiculite ore deposits may also contain a variety of other materials such as mica, quartz, and feldspar. These deposits vary from one mining location to another. During the manufacturing process, some of these materials may pose potential health hazards to workers. In the United States and many other countries, these hazards are defined in Material Safety Data Sheets (MSDS), which identify the hazard and provide information on the safe handling and disposal of the material.
One of the most common health hazards in processing vermiculite comes from quartz, which is crystalline silica. It is usually only present as larger particles, but when it is ground into finer particles, the dust can be inhaled and cause a lung disease called silicosis. As a result, strict dust control and personal protection measures are incorporated into those areas of the vermiculite-processing operation where the materials are ground, sifted, and bagged. At the consumer level, exposure to silica dust is negligible and does not pose a health hazard.
In some vermiculite ore deposits, there may also be certain amounts of various forms of asbestos. None of the ore bodies currently used by major vermiculite producers pose an asbestos health risk to workers when the material is processed in accordance with the applicable MSDS. In August 2000, the United States Environmental Protection Agency (EPA) issued a report regarding vermiculite sold as a soil amendment. In the report, they concluded there was little or no risk to consumers from asbestos.
The Future
Although there are several other materials that may be used as a substitute for vermiculite, vermiculite's extremely low density and thermal conductivity continue to make it attractive for many applications. In 1999, it was estimated there were approximately 55 million tons (50 million metric tons) of vermiculite reserves in the world.
Where to Learn More
Books
Hombostel, Caleb. "Vermiculite." In Construction Materials: Types, Uses, and Applications. New York: John Wiley & Sons, Inc., 1991.
Other
Grace Construction Products "Grace Specialty Vermiculite History." (2000). http://www.graceconstruction.com/vermiculite/verm_prodhist.html (January 2001).
Hindman, James R. "Vermiculite as an Industrial Mineral" (1997). http://www.mcn.net/~vermiculite/overview.htm (March 22, 2000).
Hindman, James R. "Vermiculite Products and Applications." (1997). http://www.mcn.net/~vermiculite/uses.htm (March 22, 2000).
Potter, Michael J. "Vermiculite." U.S. Geological Survey, Mineral Commodity Summaries, February 2000. http://minerals.usgs.gov/minerals/pubs/comodity/vermiculite/index.html (May 18, 2000).
The Schundler Company. "Basic Vermiculite Information and Data." http://www.schundler.com/techverm.htm (May 18, 2000).
The Vermiculite Association. "About Vermiculite." http://www.vermiculite.org (May 18, 2000).
— Chris Cavette
Comment about this article, ask questions, or add new information about this topic: