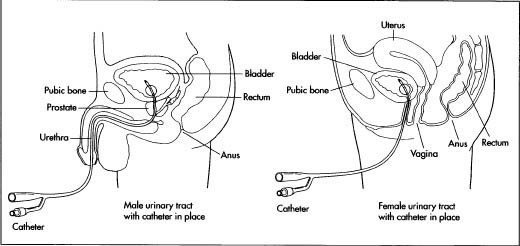
Catheter
Background
A catheter is a flexible tube made of latex, silicone, or Teflon that can be inserted into the body creating a channel for the passage of fluid or the entry of a medical device. For many years, the epidermal catheters used were plain tubes made of available industrial compounds, and design was largely based on current need. In the 1950s and early 1960s, a very common practice was to cut a suitable length of industrial polyvinyl chloride (PVC) or nylon tubing and have it sterilized with the other surgical equipment. Nowadays, there are many specialized catheter designs. For example, specific catheter designs allow catheters to be used in pulmonary, cardiac (vascular), neonatal, central nervous system, and epidural tissues. Catheters are designed to perform tissue ablation (tissue removal) and even serve as conduits for thermal, optics, and various medical devices.
The three major types of catheters are coronary, renal, and infusion. Coronary catheters are used for angiography (x-ray of blood vessels after injection of radiopaque substance), angioplasty (altering the structure of a vessel), and ultrasound procedures in the heart or in peripheral veins and arteries. The best-known renal catheters are Foley catheters, which have been commercially available since the 1930s. These catheters are equipped with an inflatable balloon at the tip and are used for urine incontinence, dying patients, and bladder drainage following surgery or an incapacitating injury or illness. The Foley catheter is relatively easy to use and used throughout the world in hospitals, nursing homes, and home-care settings.
History
The earliest precursor to the present day Foley catheter is documented in 3000 B.C. It is believed that Egyptians used metal pipes to perform bladder catheterizations. As early as 400 B.C. , hollow reeds and pipes were used in cadavers to study the form and function of cardiac valves.
In 1844, Claude Bernard inserted a mercury thermometer into the carotid artery of a horse and advanced it through the aortic valve into the left ventricle to measure blood temperature. It is because of his work that the use of catheters became the method of standard for physiologists in the study of cardiovascular blood flow. Adolph Fick took another major step in the development of cardiac catheterization in 1870. His famous note on the calculation of blood flow is the basis for today's cardiac procedures.
Among the earliest published descriptions of human catheterization were done by Frizt Bleichroeder, E. Unger, and W. Loeb in 1912. They were among the first to insert catheters into the blood vessels without x-ray visualization. Interest in catheterization was also stimulated with the advent of chemotherapy. Early chemotherapy required the injection of drugs directly into the central circulation. Bleichroeder inserted catheters into dog arteries and assessed the effects after leaving them in place for several hours. He reported no complications or clots.
The Foley catheter came into existence in the 1930s. Frederick E. B. Foley began to experiment with different catheters of the time. He realized that urinary catheters would easily slip out of the bladder because there was no way to hold them in place. Foley experimented with different methods
Raw Materials
Foley catheters are made from either silicone or latex rubber, depending on the use.
Design
Foley catheters are made of latex or silicone rubber. Silicone rubber catheters are believed to be superior to latex catheters, as silicone is more biocompatible, causes less cell death, less likely to become encrusted, and more resistant to bacterial colonization. The catheter can either have two or three outlets. In a two-way catheter, one outlet acts a urine output and the other inflates the balloon. A three-way Foley catheter has the same function as a two-way catheter, but uses the third outlet for bladder irrigation.
Foley catheters vary in size from 12 fr to 30 fr (4 to 10 mm) in diameter, with the standard being 14 fr (4.6 mm). The balloon itself varies in size from 5 cc to 30 cc, depending on the needed use. The balloon can either be filled with sterile water or air. The catheter can also be attached to a drainage bag.
The Manufacturing
Process
- The first step in the manufacturing of a Foley catheter is the production of the long, thin tube that will be inserted into the bladder. The liquid rubber silicone is poured into a room temperature vulcanization (RTV) rubber mold. The mold is shaped like the desired catheter with either two or three outputs.
- The silicone is then heat cured. This procedure can take anywhere from 0.5 to 40 hours. Once cooled, the tube is withdrawn from the mold.
- A small opening is then punched in the distal end of the tube furthest away from the two outputs.
- A thin band of cured latex is slipped over the tube by hand to form a sheath around the tube. It is positioned so that the latex covers the opening that has been punched in the tube.
-
To form the balloon, the entire length of the tube is dipped in latex,
which creates an overcoat layer and bonds the band to the tube proximate
to the distal and proximal ends of the band, forming the balloon. This
adds to the thickness of the balloon and is used to adjust the outer diameter of the tube to the desired size.
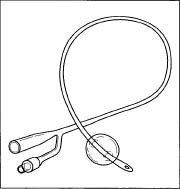 A Foley catheter.
A Foley catheter. - The catheter is then transported to the packaging center where it is put into a kit with a needleless syringe (to fill the balloon) and a drainage bag.
Quality Control
Quality control is built into each step of the manufacturing process. The machine operations check the final product of each stage in the process. The thermoplastic materials are immersed in liquid to ensure that defects are not present and that there will not be any leakage.
Byproducts/Waste
Any material deemed to be defective is either discarded or recycled depending on the severity of the damage. Since the product is directly related to human health, the materials must be of the highest quality.
The Future
A new use of the catheter is being tested in medical facilities for the purpose of dissolving clots or blockages in the coronary arteries. Once the catheter is positioned in the coronary artery, the tip of the catheter acts much like a showerhead, spraying six jets of saline around the clot. These saline streams break down the clot and the vacuum-like natures of the pumps force the debris out of the artery. With the clot gone, doctors can proceed with balloon angioplasty to repair the fatty blockage, which caused the clot to lodge there in the first place. This method requires only mild intravenous sedation rather than the general anesthesia that would be required with bypass surgery. Such new technology lessens the physical and emotional strain on a patient.
In current ablation systems (catheter used for tissue destruction), the tip of a radio frequency ablation catheter can become quite hot. Blood can subsequently form coagulum on the catheter tip that prevents delivery of successful lesions. Another advance is active cooling of the catheter tip. This allows higher energy delivery at a cooler tip temperature without an increased risk of coagulum formation. The higher energy results in a better lesion.
Catheters are also being designed with safety features to prevent needlestick injuries along with Silver/Hydrogel-Coated Foley catheters to resist bacterial infection.
The fastest growing segment of the catheter industry, the coronary catheter market, is expected to reach four billion dollars by 2003, growing at 11.2% annually. The largest segment, however, is the renal market, which is comprised primarily of urinary catheters and dialysis catheters. Currently a four billion dollar segment, it is expected to reach 7.1 billion dollars in 2003.
Where to Learn More
Books
Topol, Eric J., ed. Textbook of Interventional Cardiology. Philadelphia: W. B. Saunders Co., 1993.
Periodicals
Mueller, R., and T. Sanborn. "The History of Interventional Cardiology." American Heart Journal 129 (1995):146-172.
Other
United States Patent Web Page. December 2001. < http://www.uspto.gov.com >.
Bonny P. McClain
thank you for the information, but as a medical practitioner and inventor of a new sort of catheters(with less risks of UTI (urinary tract infection) and more patient comfort), i need more specific and detailed information about how exactly step by step a foley catheter is made. and i really appreciate if you help me have such information.
thank you