Dry Ice
Background
Dry ice is the name given to carbon dioxide when it is in a solid state. Carbon dioxide is found in the earth's atmosphere; it is a gas that humans exhale and plants use for photosynthesis. This chemical compound is colorless, odorless, tasteless, and about 1.5 times as dense as air. Carbon dioxide turns from gas to an opaque white solid while under pressure and at low temperatures, turning solid at −109°F (178.5°C). Dry ice is manufactured primarily in two forms, either as a block of dry ice which weighs over 50 lb (22.7 kg) or in small pieces that vary in size from the size of a grain of rice to a larger pellet. Dry ice does not melt, instead it sublimates, meaning the solid turns directly into a gas (bypassing the liquid state) as the temperature rises and the solid begins to dissipate. This unusual feature results in a smoking effect, and dry ice appears to be steaming as it sublimates. Thus, dry ice is often used to simulate fog or smoke.
Dry ice itself is not poisonous, but the surface of the solid is so cold that it should not be touched without gloves. Also, while the gas is stable and inert, it is heavier than air and can concentrate in low areas or enclosed spaces. When the concentration of carbon dioxide in the air exceeds 5%, the carbon dioxide becomes toxic. Thus, any area in which dry ice is used must be well ventilated.
It is relatively simple to turn carbon dioxide from a gas to a solid. Many dry ice manufacturers exist in the United States, and dry ice is shipped to all parts of the country for a variety of uses. It is an important refrigerant for keeping foods cold and preventing bacterial growth during shipment. Dry ice used for cooling or freezing foods must be very clean and considered "food grade" to ensure that food it may touch will not be contaminated. Because the solid sublimates rather than melts, large quantities of dry ice can be put into shipping containers without having to take into account volume of melting water that accumulates when ice is used as a refrigerant. Food-related uses are extensive and include quick freezing of foods for future use at food processing plants, retarding the growth of active yeast at bakeries, and keeping foods chilled for catering for the airline industry.
Other uses include: slowing the growth of flower buds at nurseries to keep plants fresh for consumers, flash freezing in the rubber industry during manufacture, absorbing ammonia refrigeration leaks, and creating smoke for theatrical productions. The most significant recent application of dry ice is dry ice blasting (or cleaning) in which dry ice pellets are hurled at a surface to be cleaned at high speed. The pellets strip the surface of the contaminants, sublimate into the atmosphere, and leave behind no toxic gases. The only residual is the dirt or paint left behind for disposal.
History
Dry ice was not invented, rather the properties of solid carbon dioxide were discovered in the early twentieth century. It was first produced commercially in the 1920s in the United States. A commercial venture trademarked the name dry ice in 1925 and solid carbon dioxide has been referred to as dry ice ever since. Until fairly recently, dry ice was often referred to as hot ice, a reference to the fact that when one touched the cold surface the hand felt burned.
It appears that the Prest-Air Devices Company of Long Island, New York first successfully produced dry ice in 1925. Also in that year, Schrafft's of New York City first used the product to keep its famous ice cream from melting inside their parlor. Dry ice was far more extensively used for refrigeration and freezing of foods in the mid-twentieth century than it is today. Virtually every ice cream parlor in the world used dry ice for keeping ice cream frozen until well after the World War II, when electric refrigeration became affordable and efficient. The manufacturing of dry ice has not changed significantly in many decades and is a relatively simple process of pressurizing and cooling gaseous carbon dioxide. Uses for dry ice have diminished somewhat with the advent of better electric refrigeration. Some recent developments for its use include using the pellets in blasting or cleaning and its increasing use in transporting medical specimens, including hearts, limbs, and tissues, for reattachment and transplantation.
Raw Materials
The only raw material used in the manufacture of dry ice is carbon dioxide. This raw material is the byproduct of the refinement of gases emitted during the manufacture or refinement of other products. Most carbon dioxide used in the manufacture of dry ice in the United States is derived from refinement of gases given off during the refinement of petroleum and ammonia. The carbon dioxide emitted during these processes is sucked off and "scrubbed" to remove impurities for food grade carbon dioxide that will eventually become dry ice.
The Manufacturing
Process
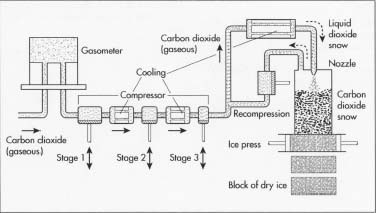
- Carbon dioxide is liquefied by compressing and cooling, liquefying at a pressure of approximately 870 lb/in 2 (395 kg/cm 2 ) at room temperature. Liquid carbon dioxide is pumped, via piping, into huge holding tanks so that dry ice manufacturers can remove the liquid required.
- The liquid carbon dioxide is shipped in huge quantities, sometimes weighing many tons. Thus, most dry ice manufacturers choose to locate their factories close to the petroleum or ammonia refineries to keep transportation costs affordable. The pressurized, refrigerated liquid carbon dioxide is piped directly into a pressurized tank or rail car owned by the dry ice manufacturer and heads for the plant.
- The tank trunk pulls up to the factory and dumps the liquid carbon dioxide into huge tanks on the premises. These tanks hold the liquid under pressure, keeping it refrigerated so that it remains in liquid state. These tanks are situated adjacent to the factory wall and, through piping, the liquid is brought directly inside when required for manufacturing.
- The liquid carbon dioxide is released, again via piping, from the adjacent tanks through the factory wall and into the dry ice press. When the liquid moves from a highly-pressurized environment to atmospheric pressure, it expands and evaporates at high speeds, causing the liquid to cool to its freezing point which is −109°F (−78.3°C). A nozzle puts the liquid into the top block of a dry ice press, which stands approximately 16 ft (4.9 m) tall. This press includes a large block at the top that can exert extreme pressure on the product that is brought into it. When the liquid carbon dioxide hits the block of the dry ice press, it immediately solidifies since it is now at room temperature. The carbon dioxide now resembles snow.
- This snow, now in the upper portion of the press, must be compressed into a block of dry ice. Thus, this top portion of the press goes up and down with extraordinary pressure (about 60 tons), squashing the snow into a solid block of dry ice. This is approximately a five minute process. When the block is solid, it is generally about 2 ft (61 cm) wide and 10 in (25 cm) high, weighing about 220 lb (100 kg).
- This block of opaque white dry ice is pushed out of the press and onto a roller. A pneumatic saw cuts the block in half and the blocks are pushed to another saw that cuts the smaller blocks yet again. Thus, the single block made in the dry ice press is now in four pieces, each weighing about 55 lb (25 kg).
-
The smaller blocks are put into containers that keep the blocks cold so
sublimation is kept to a minimum. If shipped as unwrapped pieces they
must be tightly packed in a container,
generally including four blocks, with very little air allowed inside to reduce sublimation. If a block is removed during shipping, the other blocks will quickly begin to dissipate. Many dry ice manufacturers wrap the blocks in paper using machines (it is wise not to touch the very cold surface) and send it distributors or wholesalers.
 The formation of dry ice is a series of chemical reactions.
The formation of dry ice is a series of chemical reactions.
Quality Control
Quality control issues primarily revolve around the grade of the carbon dioxide used in the manufacture of food grade dry ice. Recently, the federal government set fairly stringent standards for the purity of carbon dioxide used in the manufacture of dry ice, causing manufacturers to certify and test the liquid carbon dioxide used, as well as the purity of the manufactured product.
Other quality control issues include ensuring that tanks and equipment are working precisely. If pressure is not properly maintained, the product cannot be produced. Moving the product quickly and efficiently from cutting to storage is very important, as dry ice quickly sublimates at room temperature, thus reducing the weight and price of the salable product. Shipping must pack the blocks densely to reduce sublimation in transit as well.
Byproducts/Waste
No significant chemicals are created in the production of dry ice. Since the product is made only from carbon dioxide, when the product sublimates the gases are emitted into the atmosphere. The only detectable waste is sublimation of the product in block form, which is kept to a minimum.
The Future
While the use of dry ice in refrigeration and food storage may be diminishing, its use in other areas holds some promise. As mentioned above, house cleaners and machinery operators are interested in the small dry ice pellets for their ability to bombard a house or machine at high pressure, remove dirt or other contaminants, and then dissipate into the atmosphere. Recently a telephone company used dry ice pellets to safely clean sensitive electronic testing equipment without using dangerous solvents. Car body repair shops have discovered that applying dry ice to dents in the body can sometimes eliminate the disfiguration. Also, tests on dry ice blocks advocate dropping it into gopher holes to eradicate the pests or putting it out in the backyard to attract mosquitoes in order to keep them away from humans.
Where to Learn More
Books
Russell, Allan S. McGraw-Hill Encyclopedia of Science & Technology. Vol. 5. New York: 1997.
The Handy Science Answer Book. Second ed. Detroit: 1997.
Other
"Dry Ice." About.com Web Page. August 2001. < http://inventors.about.com/library/inventors/bldryice.htm >.
dryicelnfo.com Home Page. July 2001. < http://www.dryiceinfo.com >.
Interview with Chuck Hines. Owner of Arctic Dry Ice Company. Baltimore. 31 July 2001.
Nancy EV Bryk
Comment about this article, ask questions, or add new information about this topic: