Sleeping Pill
Background
A sleeping pill, also commonly called a sleep aid, is a drug that helps a person fall asleep or remain sleeping. Disorders such as insomnia (inability to sleep) are widespread, and drugs to induce sleep have been used since ancient times. Two distinct categories of sleeping pills are sold in the United States: prescription and over-the-counter drugs. Most prescription sleeping pills have a type of drug known as a benzodiazepine (a central nervous system depressant) as the active ingredient. Benzodiazepines include chlordiazepoxide (Librium) and diazepam (Valium). Pharmacists developed non-benzodiazepine hypnotics in the 1990s, such as zopiclone and zaleplon (Sonata). Over-the-counter sleep aids, which can be bought without a prescription, contain antihistamines. Both prescription and over-the-counter sleep aids can cause side effects, such as next-day drowsiness, and an overdose can be hazardous. The manufacturing of sleeping pills is highly regulated and overseen by the Food and Drug Administration (FDA).
History
Sleeping potions were some of the earliest drugs discovered, and sleep aids are still among the most widely used drugs today. The ancient Greeks and Egyptians used the extract of the opium poppy to induce sleep. The Greek god of sleep, Hypnos, was usually depicted holding a poppy flower. The juice of the poppy contains chemicals known as opiates, from which morphine and heroin are distilled. Ancient Greeks and Romans knew several other herbal sleep-inducers. The bark of mandrake, or mandragora, was used as a sleep aid, as were the seeds of an herb called henbane. The juice of lettuce was also used to induce sleep. As early as 300 B.C. , Greek doctors were known to prescribe concoctions of these different plant derivatives. Similar prescriptions were also apparently known throughout the Arab world. Apothecaries of the Middle Ages in Europe stocked "spongia somnifera," a sponge soaked in wine and various herbs. Other mixtures were known in England in the Middle Ages and the Renaissance as "drowsy syrups." Plant-based sleep aids were all that were available up until the nineteenth century. The chemist Frederick Setumer synthesized opium in 1805, and other advances in sleep drugs followed by the middle of the century.
Two drugs used in the nineteenth century to induce sleep were bromides and chloral hydrate. Chloral hydrate was synthesized in 1832 by a German chemist, Justus von Liebig. Chloral hydrate is a central nervous system depressant that acts very rapidly. Chloral hydrate alone could send a person into deep sleep in about half an hour. Chloral hydrate works much more quickly in combination with alcohol. Slipped into whiskey, it was the "knockout drops" of the underworld, also called a "Mickey Finn." The class of sleeping drugs called bromides were invented in 1857 by an English chemist, Sir Charles Locock. There are several bromide salts, including sodium bromide, potassium bromide, and ammonium bromide, which all act as central nervous system depressants. Locock first used bromides as an anticonvulsant to treat epileptics. A German doctor, Otto Behrend, discovered in 1864 that potassium bromide was a useful sedative. The various bromides be-came popular as sleep aids in the late nineteenth and early twentieth century.
The most popular sleeping pills of the early twentieth century were the barbiturates. The barbiturates comprise a huge class of drugs with at least 25,000 known compounds. Of these compounds, about 50 were or are marketed as prescription drugs. The forebear of the barbiturates was actually discovered in the mid-nineteenth century. A Prussian chemist, Adolf von Baeyer, is credited with inventing and naming barbituric acid in 1863 or 1864. He created the acid out of a compound of malonic acid and urea. On the day of his discovery, Baeyer is said to have gone to a nearby tavern to celebrate. Some sources say it happened to be the feast of St. Barbara that day; others say the barmaid was named Barbara. In any case, he named the compound barbituric acid. In itself, barbituric acid was useless. In 1903, a student of Baeyer's, along with another German chemist, produced a new compound out of barbituric acid and a diethyl derivative. The new chemical, given the trade name Veronal, was an excellent sedative and sleep aid. Other researchers came up with more barbituric acid derivatives. The most widely used was phenobarbital. Many European and American pharmaceutical companies came up with new barbiturates in the 1920s and 1930s. The Eli Lilly Company produced the widely used Amytal and Seconal, and Abbott Laboratories invented Pentothal.
Though the barbiturates were effective sleep aids, they also proved dangerous. Barbiturates are addictive, can have a variety of unpleasant side-effects, and their effectiveness is greatly increased when taken with alcohol. Barbiturate sleeping pills taken with alcohol can quickly bring on death, whether through accidental overdose or planned suicide. Scientists developed safer sleeping pills in the 1970s, the benzodiazepines. Early benzodiazepine drugs shared problems with barbiturates. They were addictive and had side effects such as memory impairment. Improved benzodiazepines were developed in the late 1970s and early 1980s. In the 1990s, non-benzodiazepine drugs were developed, which leave the body much more quickly than the older drugs.
The barbiturates and benzodiazepines are ingredients in prescription drugs, given out by a doctor. Until the 1970s, ingredients in over-the-counter sleep medications were not closely regulated in the United States. The FDA began reviewing over-the-counter drugs in the early 1970s, and by 1978 had approved one active ingredient for an over-the-counter sleep aid. This was the antihistamine doxylamine succinate. In 1982, the FDA approved two more antihistamines for non-prescription hypnotics. These are diphenhydramine HICI and diphenhydramine citrate. These three drugs are the only active ingredients approved for non-prescription sleep aids in the United States. Though it is possible to overdose on these drugs, they are not nearly as strong as prescription sleep aids, and in most cases can be used safely when the directions are followed.
Raw Materials
A non-prescription sleeping pill sold in the United States may contain only one of three approved active hypnotic ingredients. As stated above, these are the antihistamines diphenhydramine HICI, diphenhydramine citrate, or docylamine succinate. Some over-the-counter sleep aids also contain other active ingredients for other conditions, such as an analgesic for pain relief. In addition, sleep aids contain inactive ingredients that are used to bind the tablet, coat it, flavor it, color it, and give it the proper consistency. Some common ingredients are sugars, starches, magnesium stearate, various artificial colors, microcrystalline cellulose, and wax. Though sleeping pills are sold under a variety of brand names, many brands are virtually identical. The number of formulas used to manufacture sleeping pills in the United States is actually quite small.
Design
For over-the-counter sleep aids, design is not a terribly important element in the manufacturing process. Sleeping pills are made in a controlled and regulated environment with a limited number of approved ingredients. Chemical engineers design a formula that meets the drug maker's needs, producing a tablet of a particular color and shape, for example. The tablet's designers also need to consider how stable the drug will be over its stated shelf life and how quickly it dissolves or breaks down in the body. Manufacturers would test a new formula as well
The Manufacturing
Process
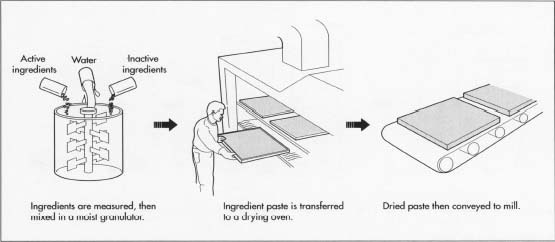
- First, workers perform what is called the "weigh up," measuring out the active and inactive ingredients for the sleep aid formula. A large manufacturer may make a batch of tons of tablets, while a smaller plant may make only several pounds at a time. In either case, workers follow the Standard Operating Procedure in measuring out the specified amounts of each ingredient. Workers are required to work in pairs, so one worker may measure and the second will verify that his partner has measured out the correct amount.
- After all the ingredients are correctly measured, they are blended. A variety of mechanical devices may be used to blend the ingredients, depending on the manufacturer and the size of the batch. Also, the blending may take part in stages. Workers may place all the ingredients in the blender at once, or groups of ingredients may be done separately. Workers then add a measured amount of water to the blender in order to wet the ingredients, again following exactly the written procedure and checking each other. The wetted powder becomes a thick paste, with the consistency of damp sand. Workers feed the paste into a hopper that has rotating hammers which crush the paste. This process is called moist granulation.
- The paste exits the moist granulator machine onto trays, forming a layer about one inch thick. The trays are next conveyed to a drying oven. The size of the oven depends on the manufacturer. It may be a small cupboard-like apparatus, or something much larger. The paste dries in the oven for several minutes to half an hour, depending on the particular formula, type of heat used, and manufacturing protocol. When dry, the material is in chunks about the size of a dime, of a brick-like hardness. The granules making up the material are coarse and uneven. In the next step, more granulation breaks the material into uniform granules.
-
Next the hardened paste is conveyed to another granulator or mill, which
breaks the paste into small particles. Various designs of machine can
perform this function. A common design uses a screen or sieve with holes
the desired size of the finished granules. A mechanical arm moving over
a series of screens forces the hardened paste to form smaller and
smaller particles. After passing through the final screen, the particles
are the correct size for compression in the tabletting machine.
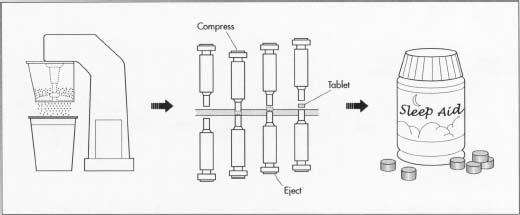 The paste is ground and formed into tablets.
The paste is ground and formed into tablets. - At this stage, the material for the sleep aid has been converted to fine granules of uniform size. Workers feed the powder into hoppers above a tabletting machine. This machine has a rotating tray of hollow dies in the shape of half the finished tablet. The powder falls from the hopper into the hollow dies. Then an arm holding an inverted die of the same shape descends and exerts several tons of pressure on the powder. This compresses the powder into tablet form. Depending on the size of the tabletting machine, it may be able to produce thousands of tablets a minute. The tablets fall onto a belt that counts them into sterile packaging. The packages are sealed, and readied for shipping.
Quality Control
Quality control occurs at every step of the sleep aid manufacturing process. All the raw ingredients, the inactive as well as the active, must be tested when they arrive at the factory, in order to make sure each is exactly what it is supposed to be. Workers follow explicit directions in carrying out each step of measuring, blending, granulating, etc., always working in pairs and checking to verify that the procedure is being carried out in the right way. A quality control inspector also follows the manufacturing process, formally "releasing" the tested ingredients so they can be mixed, testing and releasing the mixture, and releasing the final product too, if it is exactly as it is supposed to be. A portion of each batch must be taken aside and subjected to testing before the batch is released. Chemical tests might include ascertaining the correct medicinal strength of the tablet, as well as physical tests for proper texture, correct speed of disintegration, and stability of the product over its shelf life. Quality control inspectors keep complete written records. Each batch is assigned a batch number, which is imprinted on the packaging. If there is any problem later with the tablets, the manufacturer can trace when and where the batch was made.
The manufacture of sleeping pills is also regulated by the FDA. Drug manufacturers need to follow guidelines laid down in an FDA monograph on night-time sleep aids. The monograph details what ingredients are acceptable in the drug formula and how the package can be labeled. Sleep aid packages explain the indications for use and must stick to FDA-approved wording. The FDA also lays out the warnings the package must carry and the dosage. The manufacturer of an over-the-counter sleep aid must follow precise manufacturing steps laid out in federal Good Manufacturing Practice regulations. These regulations spell out what kind of facility is considered adequate for producing drugs, what kind of machinery can be used, how the machinery is to be installed and maintained, how measuring instruments are to be calibrated, and many other rules regarding the physical aspect of the plant and equipment. Good Manufacturing Practice also requires certain quality control steps be taken. Inspectors from the FDA visit the plant at least once every two years in order to ascertain that the Good Manufacturing Practice rules are being followed. A plant making sleep aids must have its Standard Operating Procedures written out and approved. The Standard Operating Procedures explain exactly how each step in the manufacturing process will be carried out. Workers must be trained to follow the Standard Operating Procedures to the letter. This prevents any variation in how the product is made. The FDA inspector will also check the actual operation of the plant against the written Standard Operating Procedures and note any discrepancies. Because of this tight control of every step of the manufacturing process, sleep aids manufactured in the United States should be of highly uniform quality.
The Future
Advances in sleep aids are likely to come from prescription drug makers. Because of the side-effects and danger of overdose of powerful sleeping pills, researchers are investigating new drugs that leave the body quicker. One of the new non-benzodiazepine sleep aids discovered in the late 1990s, zaleplon, has the advantage of leaving the body in as little as five hours. This makes it possible to treat a form insomnia where a person falls asleep, but then wakes up after a few hours, unable to fall back asleep. Drugs like this have what is called a short elimination half-life. If a new drug like this proved effective as a prescription drug, it is possible that the manufacturer would move to petition the FDA to change its status to an over-the-counter drug. Yet since the FDA issued its first monograph on over-the-counter sleep aids in 1978, it has not added any new classes of drugs to this category.
Where to Learn More
Books
Alderborn, Gooran, and Christer Nystrbom, eds. Pharmaceutical Powder Compaction Technology. New York: Marcel Dekker, Inc. 1996.
Kales, Anthony, ed. The Pharmacology of Sleep. New York: Springer-Verlag, 1995.
Periodicals
"New Hypnotic, Zaleplon, Shows Advantages for Treating Insomnia." Psychopharmacology Update (August 1998): 1.
"Over-the-Counter Sleep Aids." Consumers' Research Magazine (April 1995): 34.
Angela Woodward
Comment about this article, ask questions, or add new information about this topic: