Titanium
Background
Titanium is known as a transition metal on the periodic table of elements denoted by the symbol Ti. It is a lightweight, silver-gray material with an atomic number of 22 and an atomic weight of 47.90. It has a density of 4510 kg/m 3 , which is somewhere between the densities of aluminum and stainless steel. It has a melting point of roughly 3,032°F (1,667°C) and a boiling point of 5,948°F (3,287 C). It behaves chemically similar to zirconium and silicon. It has excellent corrosion resistance and a high strength to weight ratio.
Titanium is the fourth most abundant metal making up about 0.62% of the earth's crust. Rarely found in its pure form, titanium typically exists in minerals such as anatase, brookite, ilmenite, leucoxene, perovskite, rutile, and sphene. While titanium is relatively abundant, it continues to be expensive because it is difficult to isolate. The leading producers of titanium concentrates include Australia, Canada, China, India, Norway, South Africa, and Ukraine. In the United States, the primary titanium producing states are Florida, Idaho, New Jersey, New York, and Virginia.
Thousands of titanium alloys have been developed and these can be grouped into four main categories. Their properties depend on their basic chemical structure and the way they are manipulated during manufacture. Some elements used for making alloys include aluminum, molybdenum, cobalt, zirconium, tin, and vanadium. Alpha phase alloys have the lowest strength but are formable and weldable. Alpha plus beta alloys have high strength. Near alpha alloys have medium strength but have good creep resistance. Beta phase alloys have the highest strength of any titanium alloys but they also lack ductility.
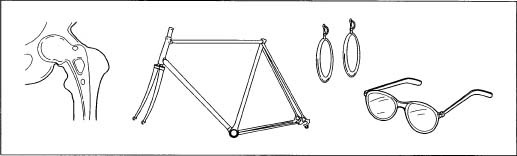
The applications of titanium and its alloys are numerous. The aerospace industry is the largest user of titanium products. It is useful for this industry because of its high strength to weight ratio and high temperature properties. It is typically used for airplane parts and fasteners. These same properties make titanium useful for the production of gas turbine engines. It is used for parts such as the compressor blades, casings, engine cowlings, and heat shields.
Since titanium has good corrosion resistance, it is an important material for the metal finishing industry. Here it is used for making heat exchanger coils, jigs, and linings. Titanium's resistance to chlorine and acid makes it an important material in chemical processing. It is used for the various pumps, valves, and heat exchangers on the chemical production line. The oil refining industry employs titanium materials for condenser tubes because of corrosion resistance. This property also makes it useful for equipment used in the desalinization process.
Titanium is used in the production of human implants because it has good compatibility with the human body. One of the most notable recent uses of titanium is in artificial hearts first implanted in a human in 2001. Other uses of titanium are in hip replacements, pacemakers, defibrillators, and elbow and hip joints.
Finally, titanium materials are used in the production of numerous consumer products. It is used in the manufacture of such things as shoes, jewelry, computers, sporting equipment, watches, and sculptures. As titanium dioxide, it is used as a white pigment in plastic, paper, and paint. It is even used as a white food coloring and as a sunscreen in cosmetic products.
History
Most historians credit William Gregor for the discovery of titanium. In 1791, he was working with menachanite (a mineral found in England) when he recognized the new element and published his results. The element was rediscovered a few years later in the ore rutile by M. H. Klaproth, a German chemist. Klaproth named the element titanium after the mythological giants, the Titans.
Both Gregor and Klaproth worked with titanium compounds. The first significant isolation of nearly pure titanium was accomplished in 1875 by Kirillov in Russia. Isolation of the pure metal was not demonstrated until 1910 when Matthew Hunter and his associates reacted titanium tetrachloride with sodium in a heated steel bomb. This process produced individual pieces of pure titanium. In the mid 1920s, a group of Dutch scientists created small wires of pure titanium by conducting a dissociation reaction on titanium tetraiodide.
These demonstrations prompted William Kroll to begin experimenting with different methods for efficiently isolating titanium. These early experiments led to the development of a process for isolating titanium by reduction with magnesium in 1937. This process, now called the Kroll process, is still the primary process for producing titanium. The first products made from titanium were introduced around the 1940s and included such things as wires, sheets, and rods.
While Kroll's work demonstrated a method for titanium production on a laboratory scale, it took nearly a decade more before it could be adapted for large-scale production. This work was conducted by the United States Bureau of Mines from 1938 to 1947 under the direction of R. S. Dean. By 1947, they had made various modifications to Kroll's process and produced nearly 2 tons of titanium metal. In 1948, DuPont opened the first large scale manufacturing operation.
This large scale manufacturing method allowed for the use of titanium as a structural material. In the 1950s, it was used primarily by the aerospace industry in the construction of aircraft. Since titanium was superior to steel for many applications, the industry grew rapidly. By 1953, annual production had reached 2 million lb (907,200 kg) and the primary customer for titanium was the United States military. In 1958, demand for titanium dropped off significantly because the military shifted its focus from manned aircraft to missiles for which steel was more appropriate. Since then, the titanium industry has had various cycles of high and low demand. Numerous new applications and industries for titanium and its alloys have been discovered over the years. Today, about 80% of titanium is used by the aerospace industry and 20% by non-aerospace industries.
Raw Materials
Titanium is obtained from various ores that occur naturally on the earth. The primary ores used for titanium production include ilmenite, leucoxene, and rutile. Other notable sources include anatase, perovskite, and sphene.
Ilmenite and leucoxene are titaniferous ores. Ilmenite (FeTiO3) contains approximately 53% titanium dioxide. Leucoxene has a similar composition but has about 90% titanium dioxide. They are found associated with hard rock deposits or in beaches and alluvial sands. Rutile is relatively pure titanium dioxide (TiO2). Anatase is another form of crystalline titanium dioxide and has just recently become a significant commercial source of titanium. They are both found primarily in beach and sand deposits.
Perovskite (CaTiO3) and sphene (CaTi-SiO5) are calcium and titanium ores. Neither of these materials are used in the commercial production of titanium because of the difficulty in removing the calcium. In the future, it is likely that perovskite may be used commercially because it contains nearly 60% titanium dioxide and only has calcium as an impurity. Sphene has silicon as a second impurity that makes it even more difficult to isolate the titanium.
In addition to the ores, other compounds used in titanium production include chlorine gas, carbon, and magnesium.
The Manufacturing
Process
Titanium is produced using the Kroll process. The steps involved include extraction, purification, sponge production, alloy creation, and forming and shaping. In the United States, many manufacturers specialize in different phases of this production. For example, there are manufacturers that just make the sponge, others that only melt and create the alloy, and still others that produce the final products. Currently, no single manufacturer completes all of these steps.
Extraction
- 1 At the start of production, the manufacturer receives titanium concentrates from mines. While rutile can be used in its natural form, ilmenite is processed to remove the iron so that it contains at least 85% titanium dioxide. These materials are put in a fluidized-bed reactor along with chlorine gas and carbon. The material is heated to 1,652°F (900°C) and the subsequent chemical reaction results in the creation of impure titanium tetrachloride (TiCl4) and carbon monoxide. Impurities are a result of the fact that pure titanium dioxide is not used at the start. Therefore the various unwanted metal chlorides that are produced must be removed.
Purification
- 2 The reacted metal is put into large distillation tanks and heated. During this step, the impurities are separated using fractional distillation and precipitation. This action removes metal chlorides including those of iron, vanadium, zirconium, silicon, and magnesium.
Production of the sponge
- 3 Next, the purified titanium tetrachloride is transferred as a liquid to a stainless steel reactor vessel. Magnesium is then added and the container is heated to about 2,012°F (1,100°C). Argon is pumped into the container so that air will be removed and contamination with oxygen or nitrogen is prevented. The magnesium reacts with the chlorine producing liquid magnesium chloride. This leaves pure titanium solid since the melting point of titanium is higher than that of the reaction.
- 4 The titanium solid is removed from the reactor by boring and then treated with water and hydrochloric acid to remove excess magnesium and magnesium chloride. The resulting solid is a porous metal called a sponge.
Alloy creation
- 5 The pure titanium sponge can then be converted into a usable alloy via a consumable-electrode arc furnace. At this point, the sponge is mixed with the various alloy additions and scrap metal. The exact proportion of sponge to alloy material is formulated in a lab prior to production. This mass is then pressed into compacts and welded together, forming a sponge electrode.
- 6 The sponge electrode is then placed in a vacuum arc furnace for melting. In this water-cooled, copper container, an electric arc is used to melt the sponge electrode to form an ingot. All of the air in the container is either removed (forming a vacuum) or the atmosphere is filled with argon to prevent contamination. Typically, the ingot is remelted one or two more times to produce a commercially acceptable ingot. In the United States, most ingots produced by this method weigh about 9,000 lb (4,082 kg) and are 30 in (76.2 cm) in diameter.
- 7 After an ingot is made, it is removed from the furnace and inspected for defects. The surface can be conditioned as required for the customer. The ingot can then be shipped to a finished goods manufacturer where it can be milled and fabricated into various products.
Byproducts/Waste
During the production of pure titanium a significant amount of magnesium chloride is produced. This material is recycled in a recycling cell immediately after it is produced. The recycling cell first separates out the magnesium metal then the chlorine gas is collected. Both of these components are reused in the production of titanium.
The Future
Future advances in titanium manufacture are likely to be found in the area of improved ingot production, the development of new alloys, the reduction in production costs, and the application to new industries. Currently, there is a need for larger ingots than can be produced by the available furnaces. Research is ongoing to develop larger furnaces that can meet these needs. Work is also being done on finding the optimal composition of various titanium alloys. Ultimately, researchers hope that specialized materials with controlled microstructures will be readily produced. Finally, researchers have been investigating different methods for titanium purification. Recently, scientists at Cambridge University announced a method for producing pure titanium directly from titanium dioxide. This could substantially reduce production costs and increase availability.
Where to Learn More
Books
Othmer, K. Encyclopedia of Chemical Technology. New York: Marcel Dekker, 1998.
U.S. Department of the Interior U.S Geological Survey. Minerals Yearbook Volume 1. Washington, DC: U.S. Government Printing Office, 1998.
Periodicals
Freemantle, M. "Titanium Extracted Directly from TiO2." Chemical and Engineering News (25 September 2000).
Eylon D. "Titanium for Energy and Industrial Applications." Metallurgical Society AIME (1987).
Other
WebElements Web Page. December 2001. < http://www.webelements.com >.
Perry Romanowski
Please advise advantages and disadvantages of seamless tubes over welded tubes.
Thanks in advance.
Please advise advantages and disadvantages of seamless tubes over welded tubes.
Thanks in advance.