Bleach
Background
Bleach is a chemical compound derived from natural sources used to whiten fabrics. Bleach works by the process of oxidation, or the alteration of a compound by the introduction of oxygen molecules. A stain is essentially a chemical compound, and the addition of bleach breaks down the molecules into smaller elements so that it separates from the fabric. Detergent and the agitation of the washing machine speed up the cleaning process. The disinfecting properties of bleach work in the same manner—germs are broken down and rendered harmless by the introduction of oxygen. In industry, different forms of bleach are used to whiten materials such as paper and wood, though most bleach is used to launder textiles.
History
Humans have been whitening fabrics for centuries; ancient Egyptians, Greeks, and Romans bleached materials. As early as 300 B.C. , soda ash, prepared from burned seaweed, was used to clean and whiten cloth. During the Middle Ages, the Dutch perfected the bleaching of fabrics in a process called crofting, whereby fabrics were spread out in large fields for maximum sunlight exposure. Textile mills as far away as Scotland shipped their material to the Netherlands for this bleaching. The practice quickly spread throughout Europe, and bleaching fields were documented in Great Britain as early as 1322. In 1728 a bleaching company using Dutch methods went into business in Galloway, Scotland. In this process, the fabrics were soaked in a lye solution for several days, then "bucked," or washed clean. The fabrics were then spread out on the grass for weeks at a time. This process was repeated five or six times until the desired whiteness was achieved. Next, the fabric was treated with sour milk or buttermilk, and again bucked and crofted. This method was lengthy and tedious, and it monopolized large tracts of land that could have been used for farming.
Late in the 18th century, scientists discovered a chemical that had the same effect as crofting, but yielded much quicker results. In 1774, Swedish chemist Karl Wilhelm Scheele discovered the chemical element chlorine, a highly irritating, green-yellowish gaseous halogen. In 1785, the French scientist Claude Berthollet found that chlorine was an excellent whitening agent in fabrics. Some mill operators attempted to expose their fabrics to chlorine gas, but the process was so cumbersome and the fumes so strong that these attempts were soon abandoned.
Near Paris, in the town of Javel, Berthollet began a small facility for the manufacture of a new product called "Eau de Javelle." The bleaching powder consisted of potash (soda ash) which had absorbed chlorine gas. In 1799, another bleaching powder was invented by Scottish chemist Charles Tennant. In the early years of the Industrial Revolution, his patented lime powder was widely used to whiten a variety of fabrics and paper products. To make the bleaching powder, slaked lime (lime treated with water) was spread thinly over the concrete or lead floor of a large room. Chlorine gas was pumped into the room to be absorbed by the lime. Though an effective whitener, the powder was chemically unstable. It was
Types of Bleach
Today, bleach is found in nearly every household. It whitens fabrics and removes stains by a chemical reaction that breaks down the undesired color into smaller particles that can be easily removed by washing. The two types of household bleach are chlorine bleach and peroxide bleach. Peroxide bleach was introduced in the 1950s. Though it helps to remove stains, especially in higher wash temperatures, it will not bleach most colored materials and does not weaken fabrics, as does sodium hypochlorite bleach. Peroxide bleach does not disinfect and is commonly added to laundry detergents which are advertised as color-safe. It also has a longer shelf life than chlorine bleach. Peroxide bleach is more commonly used in Europe, where washing machines are manufactured with inner heating coils that can raise the water temperature to the boiling point.
The more common form of household bleach in the U.S. is chlorine bleach. It is most effective in removing stains and disinfecting fabrics. Chlorine bleach is cheap to manufacture and effective in both warm and hot wash temperatures. However, it has strong chemical properties which can weaken textile fibers.
The disinfecting properties of chlorine bleach can also be useful outside the laundry. Chlorine bleach disinfects drinking water where groundwater contamination has occurred, as it is a powerful germicide. It was first used to sanitize drinking water in New York City's Croton Reservoir in 1895, and is approved by the government for sanitizing equipment in the food industry. In recent years, bleach has been promoted by community health activists as a low-cost method of disinfecting the needles of intravenous drug users.
Raw Materials
The raw materials for making household bleach are chlorine, caustic soda, and water. The chlorine and caustic soda are produced by putting direct current electricity through a sodium chloride salt solution in a process called electrolysis. Sodium chloride, common table salt, comes from either mines or underground wells. The salt is dissolved in hot water to form a salt solution, which is then treated for impurities before it is reacted in the electrolytic cell.
The Manufacturing
Process
The manufacture of sodium hypochlorite bleach requires several steps. All the steps can be carried out at one large manufacturing facility, or the chlorine and caustic soda can be shipped from different plants to the reactor site. Both chlorine and caustic soda are hazardous chemicals and are transported according to strict regulations.
Preparing the components
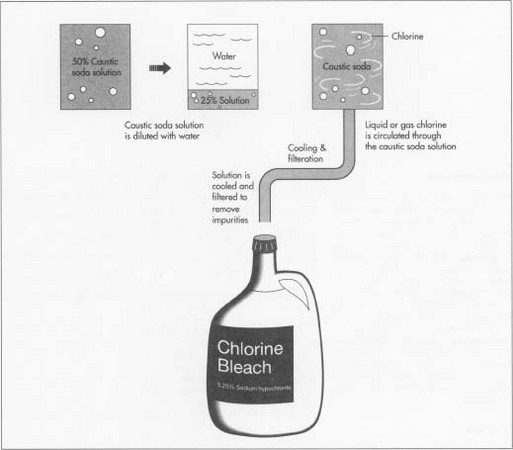
- 1 Caustic soda is usually produced and shipped as a concentrated 50% solution. At its destination, this concentrated solution is diluted with water to form a new 25% solution.
- 2 Heat is created when the water dilutes the strong caustic soda solution. The diluted caustic soda is cooled before it is reacted.
The chemical reaction
- 3 Chlorine and the caustic soda solution are reacted to form sodium hypochlorite bleach. This reaction can take place in a batch of about 14,000 gallons or in a continuous reactor. To create sodium hypochlorite, liquid or gaseous chlorine is circulated through the caustic soda solution. The reaction of chlorine and caustic soda is essentially instantaneous.
Cooling and purifying
- 4 The bleach solution is then cooled to help prevent decomposition.
- 5 Often this cooled bleach is settled or filtered to remove impurities that can discolor the bleach or catalyze its decomposition.
Shipping
- 6 The finished sodium hypochlorite bleach is shipped to a bottling plant or bottled on-site. Household-strength bleach is typically 5.25% sodium hypochlorite in an aqueous solution.
Quality Control
In the bleach manufacturing facility, the final sodium hypochlorite solution is put through a series of filters to extract any left-over impurities. It is also tested to make certain that it contains exactly 5.25% sodium hypochlorite. Safety is a primary concern at manufacturing plants because of the presence of volatile chlorine gas. When the chlorine is manufactured outside the reactor facility, it travels in liquid form in specially designed railroad tank cars with double walls that will not rupture in the event of a derailment. On arrival at the plant, the liquid chlorine is pumped from the tank cars into holding vat.. As a safety measure, the tank cars have shutoff valves that work in conjunction with a chlorine detection system. In the event of a chlorine leak, the detection system triggers a device on the tank that automatically stops the transmission of the liquid in 30 seconds.
Inside the facility, chlorine vats are housed in an enclosed area called a car barn. This enclosed room is equipped with air "scrubbers" to eliminate any escaped chlorine gas, which is harmful to humans and the environment. The vacuum-like scrubber inhales any chlorine gas from the enclosed area and injects it with caustic soda. This turns it into bleach, which is incorporated into the manufacturing process. Despite these precautions, safety and fire drills are scheduled regularly for plant personnel.
Special Considerations in
Packaging
Household sodium hypochlorite bleach was introduced to Americans in 1909 and sold in steel containers, then in glass bottles. In the early 1960s, the introduction of the plastic jug brought a cheaper, lighter, and nonbreakable packaging alternative. It reduced transportation costs and protected the safety of workers involved in its shipping and handling. Additionally, the thick plastic did not permit ultraviolet light to reach the bleach, which improved its chemical stability and effectiveness. In recent years, how-ever, plastic containers have become an environmental concern because of the time it takes the material to decompose in a landfill. Many companies that depend on plastic packaging, including bleach manufacturers, have begun to reduce the amount of plastic in their packaging or to use recycled plastics. In the early 1990s, Clorox introduced post-consumer resins (PCR) in its packaging. The newer bottles are a blend of virgin high-density polyethylene (HDPE) and 25% recycled plastic, primarily from clear milk jug-type bottles.
Consumer Safety
The bleach manufacturing industry came under fire during the 1970s when the public became concerned about the effects of household chemicals on personal health. Dioxin, a carcinogenic byproduct of chemical manufacturing, is often found in industrial products used to bleach paper and wood. In its final bottled form, common sodium hypochlorite bleach does not contain dioxins because chlorine must be in a gaseous state for dioxins to exist. However, chlorine gas can form when bleach comes into contact with acid, an ingredient in some toilet-bowl cleaners, and the labels on household bleach contain specific warnings against such combination.
In addition to the danger of dioxins, consumers have also been concerned about the toxicity of chlorine in sodium hypochlorite bleach. However, the laundry process deactivates the potentially toxic chlorine and causes the formation of salt water. After the rinse water enters the water system through the household drain, municipal water filtration plants remove the remaining traces of chlorine.
Where To Learn More
Periodicals
Ainsworth, Susan. "Resurgence in Demand Reviving Market for Sodium Chlorite." Chemical & Engineering News, March 22, 1993, pp. 11-12.
Grime, Keith and Allen Clauss. "Laundry Bleaches and Activators." Chemistry and Industry, October 15, 1990, pp. 647-49.
— Carol Brennan
The manufacturing section of this entry was written with the help of Clorox Company.
could you please help us to prevent this gas accumalation.
thanx
This is the sort of history that should be introduced into high school chemistry class to increase the interest of female students. Some of my female friends did not listen to their mothers and burned holes in their clothes trying to soak them. My mother showed me a hole burned into a cloth by improper use. My ex-fiancee, scared of bleach from his experience in the Navy, messed up my whole laundry. I would not step outside the house without bleached white clothing. It smells so fresh.
But anyway, an excellent article.
First off, I am not a chemist, but I am a Texas state licensed water/wastewater facility operator and have experience in the field since 1980.
I maintain chlorine & bleach injection systems, bottles, containers, etc daily & have had specific chemical handling training regarding those systems.
In warmer climates such as mine, Sodium Hypochlorite (bleach) in any strength from 2.5% to 12% has a reaction as it breaks down called "off-gassing" where it converts to gaseous form. If not allowed to vent, it will expand in the container and eventually explode.
It happens to me if I leave a container in the back of my truck and especially in the sun or on a hot day. My larger 300 to 500 gallon tanks don't have this problem because they have vent pipes on them.
Your solution, (provided it is not hazardous to do so) is to simply not seal the gallon container lids tightly allowing the bleach to off-gas.
I typically carry 3 to 5 one gallon bottles (store-bought) for emergency disinfection where needed and I open the screw on lid and puncture the sealed tab with a nail and put the screw on lid back on but not tightly sealed. This allows the gas to escape and the bottle doesn't swell or explode.
Only problem with this is the bleach will lose potency quickly and after a couple of weeks and in my case not even smell like bleach or work as expected.
Hope this helps
Rich