Salt
Background
Salt is the common name for the substance sodium chloride (NaCI), which occurs in the form of transparent cubic crystals. Although salt is most familiar as a food supplement, less than 5% of the salt produced in the United States is used for that purpose. About 70% is used in the chemical industry, mostly as a source of chlorine. Salt is also used for countless other purposes, such as removing snow and ice from roads, softening water, preserving food, and stabilizing soils for construction.
The earliest humans obtained their salt from natural salt concentrations, called licks, and from meat. Those people who lived near the ocean may have also obtained it by chewing seaweed or from the natural evaporation of small pools of seawater. Meat became a more important source of salt as hunting was developed, as did milk when sheep, goats, horses, camels, reindeer, and cattle were domesticated. Even today, certain peoples—such as the Inuit of the far north, the Bedouin of the Middle Eastern deserts, and the Masai of east Africa—use no other form of salt.
As agriculture developed, leading to an increased population and a diet consisting mostly of plants, it became necessary to devise ways of obtaining salt in greater amounts. The earliest method of salt production was the evaporation of seawater by the heat of the sun. This method was particularly suited to hot, arid regions near the ocean or near salty lakes and is still used in those areas. Solar evaporation was soon followed by the quarrying of exposed masses of rock salt, which quickly developed into the mining of underground deposits of salt. Two thousand years ago the Chinese began using wells to reach underground pools of salt water, some of which were more than 0.6 miles (1.0 km) deep.
In areas where the climate did not allow solar evaporation, salt water was poured on burning wood or heated rocks to boil it. The salt left behind was then scraped off. During the time of the Roman empire, shallow lead pans were used to boil salt water over open fires. In the Middle Ages these were replaced with iron pans which were heated with coal. In the 1860s a procedure known as the Michigan process or the grainer process was invented, in which salt water was heated by steam running through pipes immersed in the water. This process is still used to produce certain types of salt. By the late 1880s open pans were replaced by a series of closed pans, in a device known as a multiple-effect vacuum evaporator, which had been used in the sugar industry for about 50 years.
Today the United States is the world's largest producer of salt, followed by China, Russia, Germany, the United Kingdom, India, and France.
Raw Materials
Salt is obtained from two sources: rock salt and brine. Rock salt is simply crystallized salt, also known as halite. It is the result of the evaporation of ancient oceans millions of years ago. Large deposits of rock salt are found in the United States, Canada, Germany, eastern Europe, and China. Sometimes pressure from deep inside the Earth forces up large masses of rock salt to form salt domes. In the United States, salt domes are found along the Gulf Coast of Texas and Louisiana.
Brine is water containing a high concentration of salt. The most obvious source of brine is the ocean, but it can also be obtained from salty lakes such as the Dead Sea and from underground pools of salt water. Large deposits of brine are found in Austria, France, Germany, India, the United States, and the United Kingdom. Brine may also be artificially produced by dissolving mined rock salt or by pumping water into wells drilled into rock salt.
Natural brines always contain other substances dissolved along with salt. The most' common of these are magnesium chloride, magnesium sulfate, calcium sulfate, potassium chloride, magnesium bromide, and calcium carbonate. These substances may be as commercially valuable as the salt itself. Rock salt may be quite pure, or it may contain various amounts of these substances along with rocky impurities such as shale and quartz.
For table salt, however, additives are usually mixed in. Most table salt is iodized in order to provide the trace element iodine to the diet. This helps to prevent goiter, a disease of the thyroid gland. To supply iodine, a small amount of potassium iodide is added. Table salt also contains a small amount of various chemicals used to keep the salt from absorbing water and caking. These chemicals include magnesium carbonate, calcium silicate, calcium phosphate, magnesium silicate, and calcium carbonate.
The Manufacturing
Process
Processing rock salt
- 1 Underground salt deposits are usually discovered by prospectors searching for water or oil. When salt is detected, a diamond-tipped, hollow drill is used to take several regularly spaced core samples throughout the area. These samples are analyzed to determine if salt mining would be profitable.
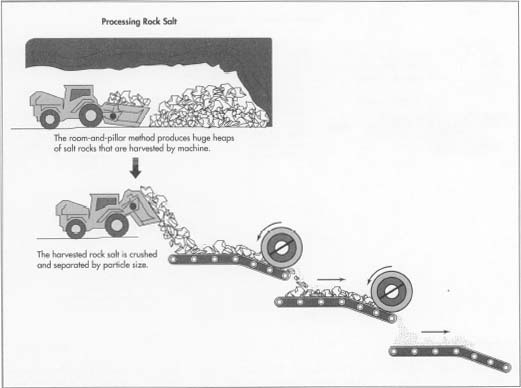
- 2 When a site is selected for mining, shafts are sunk into the center of the salt deposit. Then a machine that looks like a gigantic chain saw is used to cut a slot about 6.0 inches (15 cm) high, about 66 feet (20 m) wide, and about 10 feet (3 m) deep into the salt at floor level. This process is known as undercutting. A series of holes are drilled into the undercut salt with an electric drill containing a tungsten carbide bit. These holes are filled with an explosive such as dynamite or ammonium nitrate. Electric blasting caps connected to long wires are attached, and the explosive is detonated from a safe distance. Cutting and blasting are repeated in a pattern that leaves pillars of salt standing to support the roof of the mining area. This is known as the room-and-pillar method and is also used in coal mines.
- 3 Chunks of blasted rock salt are transported to an underground crushing area. Here they are passed over a grating known as a grizzly which collects pieces smaller than about 9 inches (23 cm). Larger pieces are crushed in a rotating cylinder between metal jaws with spiked teeth. The salt is then transported outside the mine to a secondary crushing area where a smaller grizzly and a smaller crusher reduce the particle size to about 3.2 inches (8 cm). At this point foreign matter is removed from the salt, a process known as picking. Metal is removed by magnets and other material by hand. Rocky material may also be removed in a Bradford breaker, a rotating metal drum with small holes in the bottom. Salt is dumped into the drum, breaks when it hits the bottom, and passes through the holes. Rocky matter is generally harder than salt, so it does not break and does not go through. The picked salt then goes to a tertiary crushing area, where an even smaller grizzly and crusher produce particles about 1.0 inch (2.5 cm) in size. If smaller particles are needed, the salt is passed through a grinder consisting of two metal cylinders rolling against each other. If purer salt is needed, rock salt is dissolved in water to form brine for further processing. Otherwise the crushed or ground salt is passed through screens to sort it by size, poured into bags, and shipped to the consumer.
Processing brine
-
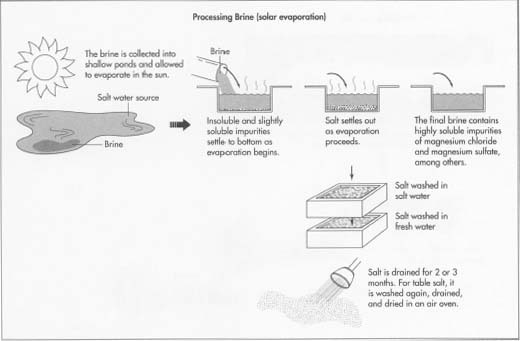
4 The simplest method of evaporating brine is solar evaporation, but it
can only
be used in hot, dry, sunny places. The brine is collected into shallow ponds and allowed to evaporate in the sun. Insoluble impurities such as sand and clay and slightly soluble impurities such as calcium carbonate settle to the bottom as evaporation begins. The brine is pumped or moved by gravity flow to another pond where calcium sulfate settles out as evaporation continues. The remaining brine is moved to yet another pond where the salt settles out as evaporation proceeds. The brine is moved one more time before evaporation is complete to prevent highly soluble impurities such as magnesium chloride, magnesium sulfate, potassium chloride, and magnesium bromide from settling out with the salt. These substances may be collected separately for commercial use.
 Rock salt is simply crystallized salt. It is the result of the evaporation of ancient oceans millions of years ago. Large deposits of rock salt are found in the United States, Canada, Germany, eastern Europe, and China.
Rock salt is simply crystallized salt. It is the result of the evaporation of ancient oceans millions of years ago. Large deposits of rock salt are found in the United States, Canada, Germany, eastern Europe, and China. - 5 The salt is scooped up by machines running on temporary railroad tracks laid on top of the layer of salt. It is then washed with highly concentrated salt water. This water contains so much salt that it cannot hold any more, so the salt is washed free of any trace impurities without dissolving. The washed salt is removed from the salt water, rinsed with a small amount of fresh water, and piled into huge stacks to drain for two or three months. At this point the salt is about 99.4% pure and can be used for many industrial purposes. If purer salt is needed, it is rewashed in salt water and fresh water, allowed to drain for one or two days, then dried in a hot air oven at about 365°F (185°C). This salt is about 99.8% pure and can be used for food processing.
-
6 Most brine is processed by a multiple-effect vacuum evaporator. This
device consists of three or more closed metal cylinders with conical
bottoms. Brine is first treated chemically to remove calcium and
magnesium compounds. It then fills the bottom
of the cylinders. The brine in the first cylinder passes through tubes heated by steam. The brine boils and its steam enters the next cylinder, where it heats the brine there. The steam from this brine heats the brine in the next cylinder, and so on. In each cylinder the condensation of steam causes the pressure inside to drop, allowing the brine to boil at a lower temperature. Salt is removed from the bottom of the cylinders as a thick slurry. It is filtered to remove excess brine, dried, and passed through screens to sort the particles by size. Salt made this way is known as vacuum pan salt and consists of small cubic crystals.
 Brine is water containing a high concentration of salt. The most obvious source of brine is the ocean, but it can also be obtained from salty lakes and underground pools of salt water.
Brine is water containing a high concentration of salt. The most obvious source of brine is the ocean, but it can also be obtained from salty lakes and underground pools of salt water. - 7 Brine may also be processed in a grainer. The brine is chemically purified and pumped into a long open pan heated by steam running through pipes immersed in the brine. The brine is heated to a temperature slightly below the boiling point and flakes of salt form on its surface as it evaporates. Usually a temperature of about 194°F (90°C) is used. Lower temperatures produce larger flakes and higher temperatures produce smaller flakes. The flakes grow until they sink to the bottom of the pan, where they are collected and dried. Grainer salt consists of small flakes rather than cubes and is preferred for certain uses in food processing. Sometimes the Alberger process is used, in which the brine is first partially evaporated in a vacuum evaporator then moved to a grainer. This process produces a mixture of flakes and cubes.
- 8 At this point salt used for most purposes is ready to be packaged in bags or boxes and shipped to consumers. To make iodized table salt, however, potassium iodide is added, then magnesium carbonate, calcium silicate, calcium phosphate, magnesium silicate, or calcium carbonate is added to make it free-flowing. The salt is then packaged and shipped to restaurants and grocery stores.
Quality Control
Specifications for salt vary widely according to the intended use. Salt intended for human consumption must be much purer than salt used for melting snow and ice, but salt used for certain scientific purposes may need to be even purer.
For most purposes, rock salt is allowed to have a gray, pink, or brown tinge rather than being pure white. The impurities that cause these colors may make up as much as 4% of a test sample. To test solubility, a 0.7-ounce (20 g) sample is placed in 6.8 fluid ounces (200 ml) of water. It should completely dissolve in no more than 20 minutes.
Evaporated salt intended for food processing is very pure, containing as much as 99.99% sodium chloride before additives are mixed in. This is important not only for safety and good taste, but because certain impurities can cause problems with certain foods. For example, small amounts of calcium tend to toughen vegetables. Traces of copper or iron tend to destroy vitamin C and to increase the rate at which fatty foods become rancid. In addition, calcium and magnesium both tend to make salt absorb more water, causing it to cake.
Health Aspects
Salt intake—or more precisely, sodium intake—is a controversial topic in health care today. Healthy adults can safely consume 0.2-0.4 ounces (6-11 g) of salt daily, which is equivalent to 0.08-0.14 ounces (2400-4400 mg) of sodium. For some people with high blood pressure, salt intake should be reduced. About one-third to one-half of all hypertensive people are salt-sensitive and will benefit from a low-sodium diet. Since there is no way to tell who these people are, most hypertensives under medical care will be placed on such a diet to see if it helps. A low-sodium diet usually aims to reduce sodium intake to less than 0.08 ounces (2400 mg) per day. While some have suggested that everyone should reduce salt intake, others point out that there is no evidence that salt restriction is of any benefit to otherwise healthy individuals.
Where To Learn More
Books
Adshead, Samuel A.M. Salt and Civilization. MacMillan, 1992.
Multhauf, Robert P. Neptune's Gift. Johns Hopkins, 1978.
Periodicals
Dornberg, John. "A 700-Year-Old Mine in Poland Is a Shrine to Human Ingenuity." Smithsonian, March 1994, pp. 96-106.
Vogel, Hans Ulrich. "The Great Well of China." Scientific American, June 1993, pp. 116-21.
Young, Gordon. "The Essence of Life: Salt." National Geographic, September 1977, pp. 380-401.
— Rose Secrest
turnips seem to have the highest sodium content 1/2 cup 40 mg
what information is available on the amount of salt absorbed while cooking vegetables - cooks tell us we just use salt to increase boiling point and cook faster - may shock us to know how little is absorbed in cooking over what is naturally there