Polyester Fleece
Background
Polyester fleece is a soft, fuzzy fabric used for sweaters, sweat shirts, jackets, mittens, hats, blankets, and in any other applications where a warm, wool-like material is needed. It is a two-sided pile material, meaning that both the front and back surface of the fabric sprouts a layer of cut fibers, similar to corduroy or velvet. Polyester fleece is an extremely durable fabric that not only holds in warmth but resists moisture and dries quickly. Unlike many other synthetic woolly textiles, polyester fleece does not pill-bunch up into little balls-after extended use. It became popular for out-door gear in the early 1990s, because back-packers and hikers found it lighter weight and warmer than wool. It is increasingly popular as a fashion fabric, and has found a host of more specialized uses. Polyester fleece has been used to make underwear for astronauts, in deep-sea diving suits, and as ear-warmers for winter-born calves.
Synthetic fibers date back to the nineteenth century, when scientists in England and Germany developed methods of extruding the liquid state of certain chemicals through fine holes, to get thread-like strings. Fiber-glass was made this way, and various other chemical fibers that were ultimately not useful as textiles. A Frenchman, Count Hilaire de Chardonnet, invented an artificial silk in the 1880s, using wood cellulose treated with nitric acid and extruded through a nozzle. Chardonnet silk was the first commercially viable synthetic fabric. In the 1920s, chemists at the Du Pont Laboratories in the United States developed nylon, an artificial fiber made of giant string-shaped molecules. British scientists extended the DuPont research in the 1940s, and came up with another polymer made of string-shaped molecules called polyester.
Polyester is made by reacting terephthalic acid, a petroleum derivative, with ethylene glycol, another petroleum derivative (commonly known as antifreeze). When the two chemicals are combined at a very high temperature, they form a new chemical known as a polymer. (Polyester is one of many chemical compounds known as polymers.) As the polymer cools, it becomes thick syrup. This syrup is forced through tiny holes in a metal disk called a spinneret. On contact with air, the streams of liquid polymer dry and harden. The crystalline structure of the polymer is a chain of interlocking molecules forming essentially giant strings. In England, this polymer was called terylene. Du Pont secured exclusive U.S. rights to the polymer in 1946, calling it polyester, with the brand name Dacron.
The chemical name for the polymer, which forms polyester, is polyethylene terephthalate, or PET. If PET is not extruded into fibers, it can be formed into the plastic commonly used for soda bottles. Interest in recycling plastics in the 1980s led to the development of polyester fiber made from used soda bottles. Many polyester fleece garments on the market today are made from a combination of recycled and virgin polyester.
Textile researchers at Malden Mills, a large manufacturer in Lawrence, Massachusetts, developed polyester fleece. Malden Mills had been the leading producer of fake fur fabric in the 1970s, but faced bankruptcy as that market softened by the end of the decade. In the 1980s, Malden's research and development department experimented with a furlike fabric made from polyester, and this with the advent of polyester fleece. Maiden began producing polyester fleece under its trade-mark names PolarTec and Polar Fleece. Maiden's brands comprise most of the polyester fleece on the market today.
Polyester fleece is extremely warm because of its structure. The pile surface provides space for air pockets between the threads, and this goes for both sides of the fabric. Because it is moisture-resistant, it can keep wearers warm even under extreme weather conditions. In the United States, the fabric was first made popular by Patagonia, a leading manufacturer of outdoor clothing and equipment. The firm marketed polyester fleece jackets to mountain climbers, and ardent customers tested the new material up and down many peaks. Other out-door clothing manufacturers followed with their own polyester fleece garment lines. Gradually the fabric crossed over from its niche as a high-tech, high-performance textile into general use.
Raw Materials
The raw material for polyester fleece is polyester, which is made from two petroleum products: terephthalic acid and ethylene glycol. Some or all of the polyester yarn may be recycled from soda bottles. Various dyes also make up raw materials, as well as finishing substances such as Teflon or other waterproofing chemicals.
The Manufacturing
Process
Producing virgin polyester
- 1 Virgin polyester—fiber that is made from reacting chemicals and not from re-used PET containers—is produced by heating terephthalic acid with ethylene glycol. Workers measure the chemicals into a vat (or in a continuous process, the chemicals may be automatically pumped in). A heating element under the vat raises the temperature of the solution to between 302-410" F (150-210° C). This first reaction creates dihydroxydiethyl terephthalate. This is then pumped into an autoclave, which is a sealed vat much like a pressure cooker. The chemical in the autoclave is heated under pressure to about 536° F (280° C). At this temperature the chemical transforms into PET. As it cools, it forms a viscous liquid. This liquid is then extruded through a showerhead-like nozzle, dried, and broken into chips.

Until the late 1800s, women wore chemises, or one-piece shifts, against their skin. Often made of linen, these shifts were not always effective in removing the perspiration that formed against the many layers of clothing worn at the time. By the 1860s, however, there was some concern that women who wore these chemises were continuously damp, thus, in cold weather, these chemise-wearers might catch a chill more easily as they might be soaked with sweat.
Prominent women's rights advocates such as Elizabeth Cady Stanton, urged women to wear "union suits." These suits, essentially a long underwear top and leggings connected at the waist, were worn closest to the skin thus replacing the chemise. They favored the union suit because the knitted suits would absorb moisture away from the skin preventing chills. Particularly favored were wool union suits—even in hot weather—because wool perhaps best draws the moisture from the body. However, short legged and short-sleeved cotton or linen suits were available for summer wear if need be, and those who could afford it might purchase silk union suits.
Nancy EV Bryk
Melt spinning
-
2 The chips of PET are next heated in another vat to 500-518° F
(260-270° C). The hot liquid is extruded through very fine
holes in a metal disk called a spinneret. As the liquid sprays out of the spinneret, it hardens into fiber form. The fibers are wound onto a heated spool. At this point, the fibers form something like a thick rope, which is called tow.
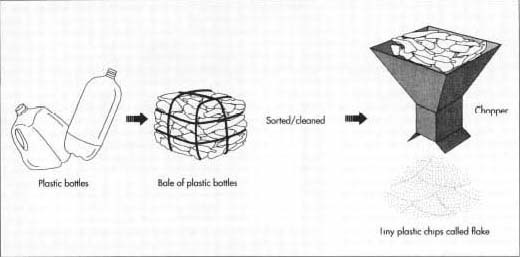 Bales of bottles are emptied onto a moving belt. Workers first sort the bottles by color, separating green ones from clear ones. Then workers visually inspect each piece so that the final result is strictly PET bottles. The sorted plastic then moves into a sterilizing bath. The clean containers are dried and crushed into tiny chips.
Bales of bottles are emptied onto a moving belt. Workers first sort the bottles by color, separating green ones from clear ones. Then workers visually inspect each piece so that the final result is strictly PET bottles. The sorted plastic then moves into a sterilizing bath. The clean containers are dried and crushed into tiny chips.
Producing polyester from recycled PET containers
-
3 When polyester is made from recycled PET, the first step is collecting
used PET containers. Yarn makers buy bales of recycled bottles from
vendors or from municipal recycling projects.
The bales of bottles are emptied onto a moving belt. Workers first sort the bottles by color, separating green ones from clear ones. Then workers visually inspect each piece, and remove anything, such as non-PET caps or bases, or any foreign objects, so that the final result is strictly PET bottles. The sorted plastic then moves into a sterilizing bath. The clean containers are dried and crushed into tiny chips. The chips are washed again, and the light-colored batch is bleached. Chips from green bottles stay green, and become yarn that will be dyed a dark color.
When the chips are thoroughly dry, they are emptied into a vat and heated, then forced through spinnerets, the same as for virgin polyester.
The finishing steps-drawing, crimping, cutting, baling-are the same as in the process for virgin polyester.
Drawing and crimping
- 4 The tow from the spool is next pulled through the heated rollers of a drawing machine to three or four times its original length. Drawing increases the strength of the fiber, and helps set the crystalline structure of the PET molecules into smooth strings. The tow then passes through a crimping machine, which compresses the tow and gives it a crinkled, accordion-like texture. This also adds strength. The crimped tow passes to a dryer, and then is cut into lengths of a few inches and baled. At this point, the short, fluffy, hairy fiber looks very much like wool.
Spinning into yarn
-
5 After the polyester is baled, a sample from each bale is inspected.
Fibers are tested for uniformity of strength and thickness. If the bale
passes inspection, then the cut tow is sent to a carding machine, which
aligns the fiber into thick, rope-like strands. The strands flow out of
the machine and are coiled into barrels or open containers. The thick
ropes are then fed into a spinning machine. The spinning machine twists
the
strand into a much finer diameter, and collects the finished yarn onto huge spools.
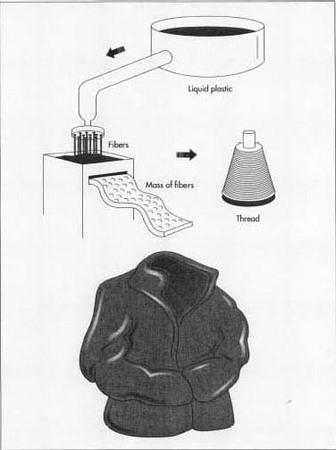 The chips are emptied into a vat and heated, then forced through spinnerets. The strands flow out of the machine and are coiled into barrels or open containers. The spinning machine twists the strand into a much finer diameter, and collects the finished yarn onto huge spools.
The chips are emptied into a vat and heated, then forced through spinnerets. The strands flow out of the machine and are coiled into barrels or open containers. The spinning machine twists the strand into a much finer diameter, and collects the finished yarn onto huge spools.
Dyeing
- 6 The textile manufacturer buys polyester from the yarn manufacturer on these spools. The yarn is next immersed in heated dye vats in the part of the factory called the dye house. In case of yarn made from green recycled PET bottles, the dye must be a dark hue. Other yarns arrive bleached white, and these can be dyed any color desired. After dyeing, workers feed the yarn through a drying machine.
Knitting
- 7 The dried yarn is next fed into a particular kind of mechanical knitter called a circular knitting machine. The knitting machine binds the yarn into a continuous tube of cloth. The tube may be approximately 58 in (1.47 m) wide and several hundred yards long.
Napping and shearing
- 8 To achieve the particular fuzzy texture of fleece, the knitted material is next fed through a napper. The napper runs mechanical bristles along the cloth, raising the surface of the textile. Next, the cloth is sent to a shearing machine, which uses a precision blade to cut the fibers raised by the action of the napper. This same process is used to make velvet, corduroy, and other textured pile fabrics.
Finishing
- 9 The fabric may next be sprayed with a waterproof material, or with some other chemical finisher that sets the texture of the material. The material is next cut into lengths, according to the customer's needs. The lengths of cloth are wrapped around boards or cardboard planks. These wound lengths are called bolts. At this point, the bolts are ready to be sent to the garment manufacturer. The manufacturer will cut the fabric according to a pattern, and sew the cloth into a garment.
Byproducts/Waste
Making polyester fleece from recycled PET bottles is a significant means to reducing the amount of plastic that is otherwise buried in landfills. One manufacturer estimates that for every meter of polyester fabric made of 80% recycled PET, eight plastic beverage bottles are kept out of landfills. Patagonia, the leading manufacturer of recycled polyester fleece garments, estimates that 25 soda bottles go into each jacket made from the fabric. Recycling PET into polyester is also alleged to be less damaging to the environment even than growing organic cotton, because cotton leaches nutrients from soil and requires so much open space to grow. The energy used to make polyester from recycled PET bottles is also significantly less than that needed to heat the chemicals for virgin polyester.
The Future
Polyester fleece is a remarkably comfortable and adaptable fabric, and will doubtless find many new uses. The future of recycled PET polyester seems to lie in making the recycling process more economically efficient, and in making finer-diameter yarns. Used beverage bottles are very lightweight, and therefore they are expensive to transport, as it takes a large volume of them to make up a ton. Yarn manufacturers must find used bottle sources near the spinning factory in order to make recycling economically viable. The coarser yarns, which are now used primarily for carpets and in tires, are easier to make, but also sell for less than the finer, garment-quality yarns. Manufacturers will continue to refine the recycling process to gain cost advantages. Other developments focus on different recycling processes that do not rely on clean soda bottles. Yarn manufacturers who recycle from PET bottles buy baled bottles from distributors. However, many municipal recycling programs do not separate PET bottles from other recyclables, and this mixed product is more difficult to handle. Several European manufacturers are developing new technology that efficiently removes excess dye, metals, and non-PET plastics from recycled PET. This means that less meticulous hand sorting is needed before the bottles are recycled. As the process is perfected, it will mean that PET and non-PET plastics can be recycled together.
Where to Learn More
Periodicals
Hamilton, Martha M. "Soda-Bottle Chic." The Washington Post (April 12,1994): Al.
Lee, Melissa. "Malden Looks Spiffy in New England Textile Gloom." The Wall Street Journal (November 10, 1995): B4.
Rotenier, Nancy. "The Golden Fleece." Forbes (May 24, 1993): 220.
Sanford, Tobey. "'Cozy, Soft, Warm, Yummy, Fleecy'-Is This Any Way to Describe an Empty Soda Bottle?" Life (November 1994): 138-140.
Schut, Jan H. "New Alchemy for PET Arrives." Plastics World (August 1995): 27-29.
— Angela Woodward
im doing a textiles portfolio for school and i need to know the fibre composition and structure of sapphire fleece.
would you be able to tell me what it consists of eg (100% cotton) and how it is knitted eg (double knit)
thanks :)
MADDI