Stirling Cycle Engine
Background
An engine is a machine that converts energy into useful work: burning coal to turn the drive shaft of a power plant generator, for example. The most common engine in production today is the gasoline-powered automobile engine. Other common engines are the diesel engine used in heavy trucks and some passenger cars, the steam turbine that generates electricity in power plants, the jet engine used to propel aircraft, and the two-stroke gasoline engine used to power smaller appliances like lawnmowers. Each of these engines converts heat generated by burning a fossil fuel into useful work.
Energy is the capacity to do work. The two quantities are related and have the same units, but energy cannot be completely converted into work. If used to fuel a stove, for example, 1 gal (3.8 1) of gasoline contains enough chemical energy to boil approximately 14 gal (53 1) of water under standard conditions. However, if that same gallon of gasoline were to be put into a portable generator (which would convert the gasoline into work and then the work into electricity) and if the electricity were then used to boil water on an electric stove, it is unlikely that more than 3 gal (11.4 1) of water could be boiled before the generator ran out of fuel.
The reason the electric stove cannot boil as much water as a gasoline-fueled stove is that engines are not 100% thermally-efficient in converting heat into work—thermal efficiency meaning the amount of useful work produced divided by the energy provided to the engine. That's why a gas range or clothes dryer is cheaper to operate than the equivalent electric appliance. In the portable generator's case, some of the gasoline's energy would end up in the engine's exhaust gases, some would be wasted heating the generator, and some would be wasted internally as the moving parts inside the generator rubbed together converting mechanical energy into frictional heat.
The science that studies how heat is cycled in an engine to create work is called thermodynamics, from the Greek therme (heat) and dynamis (power). A cycle that converts heat into work is known as a thermodynamic cycle. A gasoline-fueled automobile engine uses the Otto Cycle. A diesel-fueled engine uses the Diesel Cycle. A steam engine, or steam power plant, uses the Rankine Cycle. None of these cycles can be used to completely convert energy into work. This is because all of them have to reject heat into the environment. A power plant or steam engine has to condense steam in order to send the water back to the boiler (losing energy). An automobile engine must reject the hot exhaust gases, containing a considerable amount of energy, out the tailpipe. The most thermally-efficient practical cycle for converting heat into work is the Stirling Cycle. The Stirling Cycle is the most thermally-efficient engine because it wastes (or rejects) the least amount of heat to the environment for the amount of work it produces of any engine. An engine that uses the Stirling Cycle is known as a Stirling Cycle engine. A Stirling Cycle engine can be used to power a car, truck, or airplane, or to generate electricity. It will do this work for less energy input than a comparable Otto, Diesel, or Rankine Cycle engine could.
History
The first practical engine was the steam engine patented by James Watt in 1769. Watt's engine converted energy into work using steam from coal-fired boilers. The Watt engine consisted of a boiler, a piston contained in a cylinder, a water-cooled condenser, a water pump, piping and conduits to move the water and steam around the engine, and linkages which converted the up and down motion of the piston into circular motion on a drive shaft. The drive shaft could be put to any number of uses, such as powering a mill or pumping water out of a coal mine.
Watt's engine used a four step thermodynamic cycle to create work. The cycle began with a valve opening to allow steam under pressure to flow into the cylinder. As the steam expanded in the cylinder, it depressed the piston, producing useful work. When the piston reached the bottom of the cylinder, the valve allowing steam to enter the cylinder was closed and a valve between the cylinder and the condenser opened. Because the condenser was at a much lower pressure than the cylinder it literally sucked the steam upward into the condenser. As the steam was pulled out of the cylinder, the piston was drawn up along with the steam, returning the piston to its starting location where it was ready to create more work. Once the steam in the condenser had been completely turned back into water, the water was pumped back to the boiler where it was converted back into steam, completing the cycle.
The thermal-inefficiency in this cycle is that there is still a great deal of energy left in the steam when it is sent to the condenser. However, hardly any of this energy can be reclaimed because steam cannot not be pumped back into the boiler without performing a large amount of work on it; often more work than the heat that is lost in the condenser. The steam must be converted to water before it can be pumped to the boiler. Thus, a great deal of the heat supplied by the burning coal is lost.
The steam engine made the modern industrial world possible, but it was not without drawbacks. The mixing of cold water and steam in conjunction with primitive metallurgy led to frequent boiler explosions. The resulting loss-of-life was the motivating factor that led the Reverend Robert Stirling (in addition to being one of the foremost engineers of his day, he was also an ordained minister of the Church of Scotland) to develop an engine that used air instead of steam to drive its piston. As a by-product, the Stirling's engine was much more thermally-efficient than Watt's engine, principally because it did not require that steam be condensed during the cycle. Although Stirling's engine was much safer, the technology of the time did not allow for the manufacturing of Stirling engines of more than a few horsepower (kilowatts).
Stirling's engine never caught on in the nineteenth century. Fossil fuels were plentiful and metallurgy improved to the point where steam engines were no longer quite as hazardous. Thus, the inherent thermal-efficiency advantage of the Stirling Cycle was not enough of a motivator to overcome the significant design challenges that faced engineers wishing to build more powerful Stirling Cycle engines. In the twentieth century, the internal combustion engine—running on the Otto Cycle—dominated the industrial world because it was less expensive to construct than a Stirling Cycle engine and because fossil fuels were still reasonably priced and plentiful. However, engine designers have never forgotten that the Stirling Cycle is the most thermally-efficient possible thermodynamic cycle and have continued to design engines that utilize it. Today, Stirling Cycle engines are used to produce most of the liquefied air made in research laboratories. They are also used in weather and spy satellites and by the Swedish Navy to power some of its submarines.
Raw Materials
The Stirling Cycle engine can be made from a variety of metals. The engine block is usually made from cast ductile iron or a cast aluminum alloy (aluminum and silicon, typically). Many of the internal parts (cranks and pistons) are also made from cast ductile iron or aluminum, but some of the components that require higher strength can be fabricated out of high strength S-7 tool steel. Gaskets and seals are made out of Lexan, Neoprene, or natural rubber. The engine is filled with pressurized helium or air, which is referred to as the working fluid. The component that transfers heat from the heat source to the working fluid is required to withstand very high and constant temperatures. It can be made out of high-strength steel or a ceramic composite material such as silicon carbide (SiC).
Design
Stirling Cycle engine design is a complex fusion of thermodynamics, heat transfer analysis, vibratory analysis, mechanical dynamics, strength of materials, and machine design. Thermodynamics is used to size the engine and select the temperature at which it will operate. Heat transfer analysis is required to determine how heat will be transferred from the heat source to the working fluid and how the engine components will be designed to withstand this heat flow. Vibratory analysis is used to balance the engine for smooth operation. Mechanical dynamics is required to calculate the induced stresses in the individual engine components. Strength of materials analysis is required to determine the size of the individual components in the engine so that they can withstand the induced stress. Machine design is required to translate the thermodynamic cycle into a working engine. Each of these design requirements involves tremendous amounts of analysis.
The Stirling Cycle engine is similar to a steam engine. Both have pistons and cylinders, and both are external combustion engines as the fuel burning takes place outside the engine. The first major difference between the two engines is that the Stirling Cycle engine uses a gas (air, hydrogen, or helium, usually) instead of water and steam as the working fluid, the fluid that moves the piston and creates work. Another important difference is that the Stirling Cycle engine has two cylinders, or spaces, one for working fluid expansion and one for working fluid compression while a steam engine has only one cylinder. However, the most important difference between the two engines is that, instead of wasting its excess heat in a condenser, the Stirling Cycle engine completes its thermodynamic cycle by storing its excess heat for use in the next cycle. Because of this, the Stirling Cycle engine is not just the most thermally-efficient engine there is, it is the most thermally-efficient engine there can be. A typical automobile has a thermal efficiency of about 30%. A coal-fired power plant might be 45% efficient. A very large diesel engine might have a thermal efficiency of 50%. The theoretical maximum thermal efficiency of a Stirling Cycle engine operating at a combustion temperature of 2,500°F (1,370°C) would be about 78%. Of course, no one has been able to build a Stirling Cycle engine with anything near that thermal-efficiency. To date, engineers have not been able to overcome the significant design problems posed by the realization of Stirling's cycle.
In a steam engine, heat is applied to a boiler to create steam, which is then used to drive pistons. In a Stirling Cycle engine, heat is applied to the outside of the engine's main cylinder, which heats the air within the cylinder. This hot air expands, driving the engine's power piston. One of the major advantages of an external combustion engine over an internal combustion engine is that the working fluid in an external combustion engine is never exposed to combustion products, and thus stays much cleaner. Also, because the heat can be created in a controlled manner outside of the rapidly cycling engine, the Stirling Cycle engine produces less than 5% of the smog-creating nitrous oxides produced by an internal combustion engine for the same output of work.
The Stirling Cycle consists of four steps, just like the steam engine's Rankine cycle. However, instead of moving the working fluid from boiler to cylinder to condenser to boiler, the Stirling Cycle engine moves the working fluid from an expansion space at a high temperature to a regenerative heat exchanger to compression space at low temperature and back. The working fluid is moved because of the temperature differences between the hot and cold sides of the engine. The hot side is heated, by burning waste for example. The cold side is simply the side that is not heated, it is only cold relative to the hot side. The key to the process is the regenerative heat exchanger. It is called regenerative because it stores heat in one part of the cycle and then gives it back in the next.
Starting at the beginning of the power stroke, the four steps of the Stirling Cycle are: The working fluid is all contained within the expansion space, it adsorbs heat from the external heat source, which causes it to expand, depressing the power piston and the displacer, producing work; the power piston is stationary while the displacer, a piston that shuttles the working fluid between spaces in the engine, but does no work, moves up, pushing the working fluid from the expansion space into the compression space. On the way, most of the heat remaining in the working fluid that was not converted to work, is transferred to the regenerative heat exchanger; with the working piston fixed at the top of the main cylinder, the working fluid is compressed in the compression space back to the original volume, which requires rejecting some heat to the cold side of the engine, a source of lost heat, and thus lost thermal efficiency; the working fluid is passed back through the regenerative heat exchanger, where it reclaims a large portion of the stored heat, and into the expansion space where it is ready to be expanded again by the external heat source to perform work.
The various movements of the power piston and the displacer (at times, they move together for constant volume processes, while at other times one is stationary while the other moves for compressions and expansions) are controlled by a rhombic drive.
The Manufacturing
Process
Component manufacturing
- 1 Engine blocks and pistons are manufactured as casting. Molten steel or aluminum is poured into a hollow mold that is shaped like the final desired product and allowed to cool. A Stirling Cycle engine block requires room for two piston cylinders, one for the power piston and one for the displacer piston (which moves the working fluid back into the power cylinder), a regenerative heat exchanger, a crankshaft, a combustion chamber, and various passageways for the working fluid to move back and forth between the two cylinders.
-
2 Once the casting has cooled, any extraneous material is ground off of
it. It is usually necessary to finish engine blocks by drilling out
holes that could not be economically cast (because of their size or
complex geometry) and reaming out the cylinders to the final desired
diameter. Reaming is necessary
because of the fine tolerance required between the piston and cylinder.
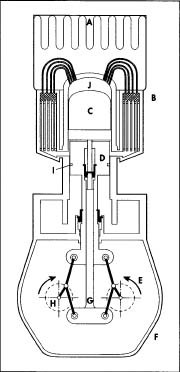 A. Heat source. B. Regenerative heat exchanger. C. Displacer. D. Working piston. E. Rhombic drive. F. Crank case. G. Displacer connecting rod. H. Crankshaft. 1. Gas seals. J. Expansion space where working fluid is heated.
A. Heat source. B. Regenerative heat exchanger. C. Displacer. D. Working piston. E. Rhombic drive. F. Crank case. G. Displacer connecting rod. H. Crankshaft. 1. Gas seals. J. Expansion space where working fluid is heated. - 3 The crankshaft and connecting rods are manufactured by forging or casting. In forging, a piece of metal stock—called a billet—is placed between two dies (dies are molds made out of very high strength tool steel). A hammer, weighing several tons, is then dropped onto the dies. The billet is usually heated almost to the melting point prior to forging. The forging process may be performed in several steps using different dies to obtain the final shape. A casting is made by filling a hollow mold with molten metal. The metal can be ductile iron, steel, aluminum, or an alloy.
- 4 The engine components are machined to achieve the final tolerances. The final shaping is usually done on a lathe. The crankshaft/connecting rod/piston is spun while cutters (made of a harder material than the part being machined) are advanced onto the spinning part to remove excess metal. Modern, computer-controlled lathes can easily attain tolerances of 0.0001 in (0.0025 mm). A milling machine, in which the part is stationary and the cutting tool rotates, is used to cut any required holes, slots, or channels in the final part.
- 5 The regenerative heat exchanger is manufactured by inserting thousands of fine steel wires through a steel plate. As the working fluid moves from the main cylinder to the auxiliary cylinder, it gives up its heat to these pins. The pins closest to the main cylinder will be the hottest. The pins closest to the auxiliary cylinder will be the coolest.
- 6 Any heat source, solar or combustion, can be used to power a Stirling Cycle engine. Regardless of source, the heat is concentrated in a chamber directly adjacent to the working piston. The cycling of the engine removes some of this heat and coverts it to work. Because the Stirling Cycle engine is an external combustion engine, the input heat can be constant (unlike the heat generated in an internal combustion engine that is produced in a series of explosions). However, because the heat is constant, the engine components in contact with the heat source must be designed to accommodate high temperatures for a long period of time.
Assembly
- 7 The crankshaft is inserted into the engine block and held there with bearings. Bearings allow the crankshaft to turn within the engine block without generating excessive frictional heat. The bearings are fixed to the engine block by pressing (the outside diameter of the bearing is slightly larger than the inside diameter of the hole in the engine block). By forcing the bearing into the engine block, the bearing is firmly fixed to the block.
- 8 The pistons and connecting rods are dropped into the cylinders and attached to the crankshaft from below using high strength bolts and lockwashers. The bolts are tightened using a predetermined torque.
- 9 The regenerative heat exchanger is inserted into the conduit that flows between the main cylinder and the auxiliary cylinder and bolted in place.
- 10 The cylinder heads are bolted to the top of the engine, an access cover is bolted to the bottom of the engine. Gaskets are used between the engine block and the covers to provide an effective seal. The heat source chamber is built into the main cylinder cover.
- 11 The working fluid is pumped into the engine. The working fluid is usually pressurized helium.
Byproducts/Waste
The Stirling Cycle engine produces much more useful work than does an internal combustion engine for the amount of greenhouse gases and smog-producing chemicals it emits. The engine can also be used to reclaim heat that would otherwise be wasted, such as landfill gas that it simply burned to get rid of it. Thus, on the whole, the engine is environmentally friendly. By harnessing solar heat in Stirling Cycle engines, electricity can be produced in areas without access to the electrical grid without the need for photovoltaic cells.
The Future
The future of the Stirling Cycle engine is very bright. If engineers can design and mass-produce a small, reliable Stirling Cycle engine, there would be no need for nuclear power or fossil-fuel burning power plants. Most of the electrical power used in homes could be generated on the premises. The engine could cool the house in the summer without use of ozone-depleting refrigerants and heat it in the winter. Unfortunately, there are serious practical design difficulties that must be overcome before the Stirling Cycle engine can come into wide use. The most significant engineering obstacle is the design of the engine combustion chamber. Because the Stirling Cycle engine operates at very high temperatures, the combustion chamber cannot be built out of the same inexpensive materials used to produce automobile engines. The use of high strength stainless steel or ceramic composites, in addition to being expensive, make manufacturing of the engine extremely difficult. Other non-trivial design obstacles include designing a reliable gearing mechanism to translate the Stirling Cycle piston motions (which are very complex compared to a standard Otto Cycle automobile engine) into crankshaft motion and designing seals capable of keeping the working fluid contained within the engine.
Where to Learn More
Books
Moran, Michael J., and Howard N. Shapiro. Fundamentals of Engineering Thermodynamics. 4th ed. John Wiley and Sons, 2000.
Organ, A. J. Thermodynamics and Gas Dynamics of the Stirling Machine. Cambridge University Press, 1992.
Walker, Graham. Stirling Engines. Oxford University Press, 1980.
Walker, Graham, Graham Reader, Owen R. Faubel, and Edward Bingham. The Stirling Alternative, Power Systems, Refrigerants and Heat Pumps. Gordon and Breach Science Publishers, 1996.
Other
Griessel, Eugene. Home Page. "Animation of a Stirling Cycle." 27 September 2001. < http://www.dynagen.co.za/eugene/stirlinghtm >.
"Stirling Cycle Frequently Asked Questions." American Stirling Company Web Page. 27 September 2001. < http://www.stirlingengine.com/FAQ.asp >.
Jeff Raines
I have a question about efficiency in heating a house. Lets say you use natural gas of LP gas in a furnace to heat your house. My thought is, instead of the furnace, what if you were to run a internal combustion engine, you capture all the heat of combustion, friction and the like and in addtion you also run a direct couple heatpump and generator combo with the crankshaft output. The question... Can you get a gain of more than 100% of the energy, then the rated btu output of gas burning alone?