Battery
Background
Benjamin Franklin's famous experiment to attract electricity by flying a kite in a lightning storm was only one of many late eighteenth- and early nineteenth-century experiments conducted to learn about electricity. The first battery was constructed in 1800 by Italian Alessandro Volta. The so-called voltaic pile consisted of alternating discs of silver and zinc separated by leather or pasteboard that had been soaked in salt water, lye, or some alkaline solution. Strips of metal at each end of the pile were connected to small cups filled with mercury. When Volta touched both cups of mercury with his fingers, he received an electric shock; the more discs he assembled, the greater the jolt he received.
Volta's discovery led to further experimentation. In 1813, Sir Humphrey Davy constructed a pile with 2,000 pairs of discs in the basement of the Royal Institution of London. Among other applications, Davy used the electricity he produced for electrolysis—catalyzing chemical reactions by passing a current through substances (Davy separated sodium and potassium from compounds). Only a few years later, Michael Faraday discovered the principle of electromagnetic induction, using a magnet to induce electricity in a coiled wire. This technique is at the heart of the dynamos used to produce electricity in power plants today. (While a dynamo produces alternating current (AC) in which the flow of electricity shifts direction regularly, batteries produce direct current (DC) that flows in one direction only.) A lead-acid cell capable of producing a very large amount of current, the forerunner of today's automobile battery, was devised in 1859 by Frenchman Gaston Planté.
In the United States, Thomas Edison was experimenting with electricity from both batteries and dynamos to power the light bulb, which began to spread in the United States in the early 1880s. During the 1860s, Georges Leclanché invented the wet cell, which, though heavy because of its liquid components, could be sold and used commercially. By the 1870s and 1880s, the Leclanché cell was being produced using dry materials and was used for a number of tasks, including providing power for Alexander Graham Bell's telephone and for the newly-invented flashlight. Batteries were subsequently called upon to provide power for many other inventions, such as the radio, which became hugely popular in the years following World War I. Today, more than twenty billion power cells are sold throughout the world each year, and each American uses approximately 27 batteries annually.
Design
All batteries utilize similar procedures to create electricity; however, variations in materials and construction have produced different types of batteries. Strictly speaking, what is commonly termed a battery is actually a group of linked cells. The following is a simplified description of how a battery works.
Two important parts of any cell are the anode and the cathode. The cathode is a metal that is combined, naturally or in the laboratory, with oxygen—the combination is called an oxide. Iron oxide (rust), although too fragile to use in a battery, is perhaps the most familiar oxide. Some other oxides are actually strong enough to be worked (cut, bent, shaped, molded, and so on) and used in a cell. The anode is a metal that would oxidize if it were allowed to and, other things being equal, is more likely to oxidize than the metal that forms part of the cathode.
A cell produces electricity when one end of a cathode and one end of an anode are placed into a third substance that can conduct electricity, while their other ends are connected. The anode draws oxygen atoms toward it, thereby creating an electric flow. If there is a switch in the circuit (similar to any wall or lamp switch), the circuit is not complete and electricity cannot flow unless the switch is in the closed position. If, in addition to the switch, there is something else in the circuit, such as a light bulb, the bulb will light from the friction of the electrons moving through it.
The third substance into which the anode and the cathode are placed is called an electrolyte. In many cases this material is a chemical combination that has the property of being alkaline. Thus, an alkaline battery is one that makes use of an alkaline electrolyte. A cell will not produce electricity by itself unless it is placed in a circuit that has been rendered complete by a simple switch, or by some other switching connection in the appliance using the battery.
Designing a cell can lead to many variations in type and structure. Not all electrolytes, for example, are alkaline. Additionally, the container for the electrolyte can act as both a container and either the cathode or the anode. Some cells draw their oxygen not from a cathode but right out of the air. Changes in the compositions of the anode and the cathode will provide more or less electricity. Precise adjustment of all of the materials used in a cell can affect the amount of electricity that can be produced, the rate of production, the voltage at which electricity is delivered through the lifetime of the cell, and the cell's ability to function at different temperatures.
All of these possibilities do, in fact, exist, and their various applications have produced the many different types of batteries available today (lithium, mercury, and so on). For years, however, the most common cell has been the 1.5 volt alkaline battery.
Different batteries function better in different circumstances. The alkaline 1.5 volt cell is ideal for photographic equipment, handheld computers and calculators, toys, tape recorders, and other "high drain" uses; it is also good in low temperatures. This cell has a sloping discharge characteristic—it loses power gradually, rather than ceasing to produce electricity suddenly—and will lose perhaps four percent of its power per year if left unused on a shelf.
Other types of batteries include a lithium/manganese dioxide battery, which has a flat discharge characteristic—it provides approximately the same amount of power at the beginning of its life as at the end—and can be used where there is a need for small, high-power batteries (smoke alarms, cameras, memory backups on computers, and so on). Hearing aids, pagers, and some other types of medical equipment frequently use zinc air button type batteries, which provide a high energy density on continuous discharge. A mercury battery is frequently used in many of the same applications as the zinc air battery, because it, too, provides a steady output voltage.
Raw Materials
This section, as well as the following section, will focus on alkaline batteries. In an alkaline battery, the cylinder that contains the cells is made of nickel-plated steel. It is lined with a separator that divides the cathode from the anode and is made of either layered paper or a porous synthetic material. The canister is sealed at one end with an asphalt or epoxy sealant that underlies a steel plate, and at the other with a brass nail driven through the cylinder. This nail is welded to a metal end cap and passed through an exterior plastic seal. Inside the cylinder, the cathode consists of a mixture of manganese dioxide, graphite, and a potassium hydroxide solution; the anode comprises zinc powder and a potassium hydroxide electrolyte.
The Manufacturing
Process
The cathode
-
1 In an alkaline battery, the cathode actually doubles as part of the
container. Huge loads of the constituent ingredients—manganese
dioxide, carbon black (graphite), and an electrolyte (potassium
hydroxide in solution)—are
delivered by train and mixed in very large batches at the production site. The mixture is then granulated and pressed or compacted into hollow cylinders called preforms. Depending on the size of the battery being made, several preforms may be stacked one on top of another in a battery. Alternatively, the series of preforms can be replaced by an extruded ring of the same material.
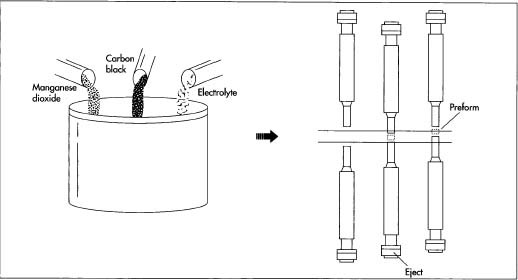 Mixing the constituent ingredients is the first step in battery manufacture. After granulation, the mixture is then pressed or compacted into preforms—hollow cylinders. The principle involved in compaction is simple: a steel punch descends into a cavity and compacts the mixture. As it retracts, a punch from below rises to eject the compacted preform.
Mixing the constituent ingredients is the first step in battery manufacture. After granulation, the mixture is then pressed or compacted into preforms—hollow cylinders. The principle involved in compaction is simple: a steel punch descends into a cavity and compacts the mixture. As it retracts, a punch from below rises to eject the compacted preform. - 2 The preforms are next inserted into a nickel-plated steel can; the combination of the preforms and the steel can make up the cathode of the battery. In a large operation, the cans are made at the battery factory using standard cutting and forming techniques. An indentation is made near the top of the can, and an asphalt or epoxy sealant is placed above the indentation to protect against leakage.
The separator
- 3 A paper separator soaked in the electrolyte solution is then inserted inside the can against the preforms; the separator is made from several pieces of paper laid at crossgrains to each other (like plywood). Looking down at an open can, one would see what looks like a paper cup inserted into the can. The separator keeps the cathode material from coming into contact with the anode material. As an alternative, a manufacturer might use a porous synthetic fiber for the same purpose.
The anode
- 4 The anode goes into the battery can next. It is a gel composed primarily of zinc powder, along with other materials including a potassium hydroxide electrolyte. This gel has the consistency of a very thick paste. Rather than a solution, it is chemically a suspension, in which particles do not settle (though an appropriate filter could separate them). The gel does not fill the can to the top so as to allow space for the chemical reactions that will occur once the battery is put into use.
The seals
-
5 Though the battery is able to produce electricity at this point, an
open cell is not practical and would exhaust its potential rapidly. The
battery needs to be sealed with three connected components. The first, a
brass "nail" or long spike, is inserted into the middle of
the can, through the gel material and serves as a "current
collector." The second is a plastic seal and the third a metal
end cap. The nail, which extends about two-thirds
of the way into the can, is welded to the metal end cap and then passed through the plastic seal.
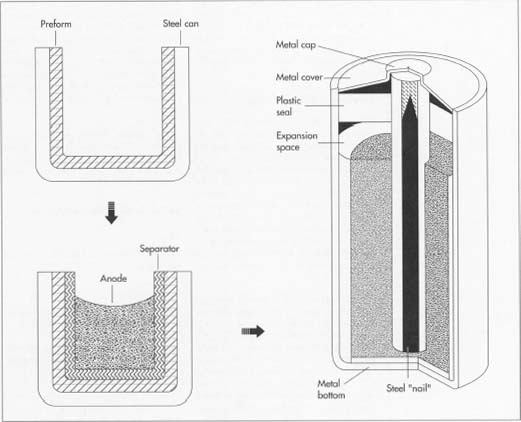 The container of a typical alkaline battery, consisting of preform inserted into a steel can, also doubles as the cathode. The anode in the middle is a gel composed primarily of zinc powder. The separator between the anode and cathode is either paper or synthetic fiber that has been soaked in an electrolyte solution.
The container of a typical alkaline battery, consisting of preform inserted into a steel can, also doubles as the cathode. The anode in the middle is a gel composed primarily of zinc powder. The separator between the anode and cathode is either paper or synthetic fiber that has been soaked in an electrolyte solution.
In the finished battery, a plastic seal, a steel nail, and a metal top and bottom have been added. The nail is welded to the metal bottom and extends about two-thirds of the way into the can, through the anode. - 6 This seal is significantly thinner in some places than in others, so that if too much gas builds up in the can, the seal will rupture rather than the entire battery. Some battery designs make use of a wax-filled hole in the plastic; excess gas pushes through the wax rather than rupturing the battery. The seal assembly meets the indentation made in the can at the beginning of the process and is crimped in place.
- 7 The opposite end of the can (the positive end of the battery) is then closed with a steel plate that is either welded in place or glued with an epoxy-type cement.
The label
- 8 Before the battery leaves the factory, a label is added identifying the type of battery, its size, and other information. The label is often paper that is simply glued to the battery. One large manufacturer has its label design printed on plastic shrink wrap: a loose fitting piece of heat-sensitive plastic is wrapped around the battery can and then exposed to a blast of heat that makes the plastic shrink down to fit tightly around the can.
Quality Control
Because battery technology is not especially new or exotic, quality control and its results are especially important as the basis for brand competition. The ability of a battery to resist corrosion, to operate well under a variety of conditions, to maintain a good shelf and usage life, and other factors, are the direct results of quality control. Batteries and ingredients are inspected and tested at almost all stages of the production process, and the completed batches are subjected to stringent tests.
Environmental Issues
Although making batteries does present some environmental obstacles, none are insurmountable. Zinc and manganese, the major chemicals in alkaline batteries, do not pose environmental difficulties, and both are considered safe by the Food and Drug Administration (FDA). The major potential pollutant in batteries is mercury, which commonly accompanies zinc and which was for many years added to alkaline batteries to aid conductivity and to prevent corrosion. In the mid-1980s, alkaline batteries commonly contained between five and seven percent mercury.
When it became apparent several years ago that mercury was an environmental hazard, manufacturers began seeking ways to produce efficient batteries without it. The primary method of doing this focuses on better purity control of ingredients. Today's alkaline batteries may contain approximately .025 percent mercury. Batteries with no added mercury at all (it is a naturally occurring element, so it would be difficult to guarantee a product free of even trace qualities) are available from some manufacturers and will be the industry-wide rule rather than the exception by the end of 1993.
The Future
Batteries are currently the focus of intense investigation by scientists and engineers around the world. The reason is simple: several key innovations depend on the creation of better batteries. Viable electric automobiles and portable electronic devices that can operate for long periods of time without needing to be recharged must wait until more lightweight and more powerful batteries are developed. Typical lead-acid batteries currently used in automobiles, for instance, are too bulky and cannot store enough electricity to be used in electric automobiles. Lithium batteries, while lightweight and powerful, are prone to leaking and catching fire.
In early 1993, scientists at Arizona State University announced that they had designed a new class of electrolytes by dissolving polypropylene oxide and polyethylene oxide into a lithium salt solution. The new electrolytes appear to be highly conductive and more stable than typical lithium electrolytes, and researchers are now trying to build prototype batteries that use the promising substances.
In the meantime, several manufacturers are developing larger, more powerful nickel-metal hydride batteries for use in portable computers. These new batteries are expected to appear in late 1994.
Where To Learn More
Books
Packaged Power. Duracell International Inc., 1981.
Periodicals
"Plastic May Recharge Battery's Future," Design News. November 17, 1986, p. 24.
Greenberg, Jeff. "Packing Power: Subnotebook Batteries, Power Management," PC Magazine. October 27, 1992, p. 113.
Leventon, William. "The Charge Toward a Better Battery: Designing a Long-Life Battery," Design News. November 23, 1992, p. 91.
Methvin, Dave. "Battery Contenders Face-Off in Struggle To Dominate Market," PC Week. November 12, 1990, p. S21.
Schmidt, K. F. "Rubbery Conductors Aim at Better Batteries," Science News. March 13, 1993, p. 166.
Zimmerman, Michael R. "Better Batteries on the Way: Nickel-Metal Hydride Is Short-Term Winner," PC Week. April 26, 1993, p. 25.
— Lawrence H. Berlow
What will be the rest Machine ? What will be the raw materials ? Is there any necessity of Gas for furnace or any other heat treatment ?