Soap
Background
Soap is a combination of animal fat or plant oil and caustic soda. When dissolved in water, it breaks dirt away from surfaces. Through the ages soap has been used to cleanse, to cure skin sores, to dye hair, and as a salve or skin ointment. But today we generally use soap as a cleanser or perfume.
The exact origins of soap are unknown, though Roman sources claim it dates back to at least 600 B.C. , when Phoenicians prepared it from goat's tallow and wood ash. Soap was also made by the Celts, ancient inhabitants of Britain. Soap was used widely throughout the Roman empire, primarily as a medicine. Mention of soap as a cleanser does not appear until the second century A.D. By the eighth century, soap was common in France, Italy, and Spain, but it was rarely used in the rest of Europe until as late as the 17th century.
Manufacture of soap began in England around the end of the 12th century. Soap-makers had to pay a heavy tax on all the soap they produced. The tax collector locked the lids on soap boiling pans every night to prevent illegal soap manufacture after hours. Because of the high tax, soap was a luxury item, and it did not come into common use in England until after the tax was repealed in 1853. In the 19th century, soap was affordable and popular throughout Europe.
Early soap manufacturers simply boiled a solution of wood ash and animal fat. A foam substance formed at the top of the pot. When cooled, it hardened into soap. Around 1790, French soapmaker Nicolas Leblanc developed a method of extracting caustic soda (sodium hydroxide) from common table salt (sodium chloride), replacing the wood ash element of soap. The French chemist Eugene-Michel Chevreul put the soap-forming process (called in English saponification) into concrete chemical terms in 1823. In saponification, the animal fat, which is chemically neutral, splits into fatty acids, which react with alkali carbonates to form soap, leaving glycerin as a byproduct. Soap was made with industrial processes by the end of the 19th century, though people in rural areas, such as the pioneers in the western United States, continued to make soap at home.
Raw Materials
Soap requires two major raw materials: fat and alkali. The alkali most commonly used today is sodium hydroxide. Potassium hydroxide can also be used. Potassium-based soap creates a more water-soluble product than sodium-based soap, and so it is called "soft soap." Soft soap, alone or in combination with sodium-based soap, is commonly used in shaving products.
Animal fat in the past was obtained directly from a slaughterhouse. Modern soapmakers use fat that has been processed into fatty acids. This eliminates many impurities, and it produces as a byproduct water instead of glycerin. Many vegetable fats, including olive oil, palm kernel oil, and coconut oil, are also used in soap making.
Additives are used to enhance the color, texture, and scent of soap. Fragrances and perfumes are added to the soap mixture to
The Manufacturing
Process
The kettle method of making soap is still used today by small soap manufacturing companies. This process takes from four to eleven days to complete, and the quality of each batch is inconsistent due to the variety of oils used. Around 1940, engineers and scientists developed a more efficient manufacturing process, called the continuous process. This procedure is employed by large soap manufacturing companies all around the world today. Exactly as the name states, in the continuous process soap is produced continuously, rather than one batch at a time. Technicians have more control of the production in the continuous process, and the steps are much quicker than in the kettle method—it takes only about six hours to complete a batch of soap.
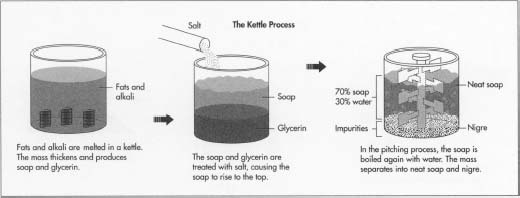
The Kettle Process
Boiling
- 1 Fats and alkali are melted in a kettle, which is a steel tank that can stand three stories high and hold several thousand pounds of material. Steam coils within the kettle heat the batch and bring it to a boil. After boiling, the mass thickens as the fat reacts with the alkali, producing soap and glycerin.
Salting
- 2 The soap and glycerin must now be separated. The mixture is treated with salt, causing the soap to rise to the top and the glycerin to settle to the bottom. The glycerin is extracted from the bottom of the kettle.
Strong change
- 3 To remove the small amounts of fat that have not saponified, a strong caustic solution is added to the kettle. This step in the process is called "strong change." The mass is brought to a boil again, and the last of the fat turns to soap. The batch may be given another salt treatment at this time, or the manufacturer may proceed to the next step.
Pitching
-
4 The next step is called "pitching." The soap in the
kettle is boiled again with added water. The mass eventually separates
into two layers. The top layer is called "neat soap,"
which is about 70% soap and 30% water. The lower layer, called
"nigre," contains most of the impurities in the soap such
as dirt and salt, as well as most of the water. The neat soap is taken
off the top. The soap is then cooled. The finishing process is the
same as for soap made by the continuous process.
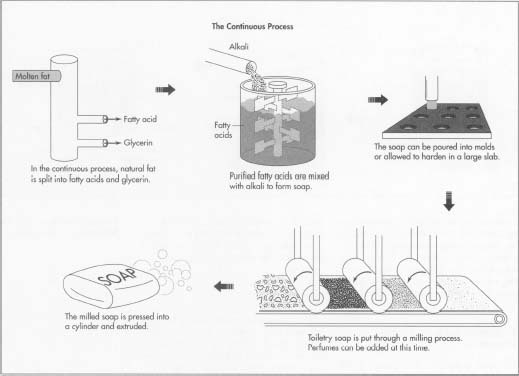 Developed around 1940 and used by today's major soap-making companies, the above illustrations show the continuous process of making soap.
Developed around 1940 and used by today's major soap-making companies, the above illustrations show the continuous process of making soap.
The Continuous Process
Splitting
- 1 The first step of the continuous process splits natural fat into fatty acids and glycerin. The equipment used is a vertical stainless steel column with the diameter of a barrel called a hydrolizer. It may be as tall as 80 feet (24 m). Pumps and meters attached to the column allow precise measurements and control of the process. Molten fat is pumped into one end of the column, while at the other end water at high temperature (266°F [130°C]) and pressure is introduced. This splits the fat into its two components. The fatty acid and glycerin are pumped out continuously as more fat and water enter. The fatty acids are then distilled for purification.
Mixing
- 2 The purified fatty acids are next mixed with a precise amount of alkali to form soap. Other ingredients such as abrasives and fragrance are also mixed in. The hot liquid soap may be then whipped to incorporate air.
Cooling and finishing
- 3 The soap may be poured into molds and allowed to harden into a large slab. It may also be cooled in a special freezer. The slab is cut into smaller pieces of bar size, which are then stamped and wrapped. The entire continuous process, from splitting to finishing, can be accomplished in several hours.
Milling
- 4 Most toiletry soap undergoes additional processing called milling. The milled bar lathers up better and has a finer consistency than non-milled soap. The cooled soap is fed through several sets of heavy rollers (mills), which crush and knead it. Perfumes can best be incorporated at this time because their volatile oils do not evaporate in the cold mixture. After the soap emerges from the mills, it is pressed into a smooth cylinder and extruded. The extruded soap is cut into bar size, stamped and wrapped.
Byproducts
Glycerin is a very useful byproduct of soap manufacture. It is used to make hand lotion, drugs, and nitroglycerin, the main component of explosives such as dynamite.
Where To Learn More
Books
Cavitch, Susan M. The Natural Soap Book: Making Herbal and Vegetable-Based Soaps. Storey Communications, 1995.
Maine, Sandy. The Soap Book: Simple Herbal Recipes. Interweave Press, 1995.
Spitz, Luis, ed. Soap Technologies in the 1990s. American Oil Chemists Society, 1990.
Other
About Soap. Procter & Gamble, 1990. (513) 983-1100.
— Sheila Dow
I do not know much about making soap, which method you will be using or how much you will make, but I was able to find basic measurements that might help you.
12 oz or 340 grams 100% lye
605 grams ice cold or part frozen distilled water
2.48 kg lard or all vegetable shortening
Good luck, Lacey J.